The hydride ion ( H^ - ) is stronger base than OH^ - ion. Which of the following reaction will occurs if sodium hydride (NaH) is dissolved in water?

The hydride ion, H^- is a stronger base than its hydroxide ion OH^- . Which of the following reactions will occur, if sodium hydride (NaH) is dissolved in water?
Solved] Sodium hydride (NaH) is a very strong base. When it is added to the compound shown below, hydrogen gas is produced as a by-product along wit... | Course Hero
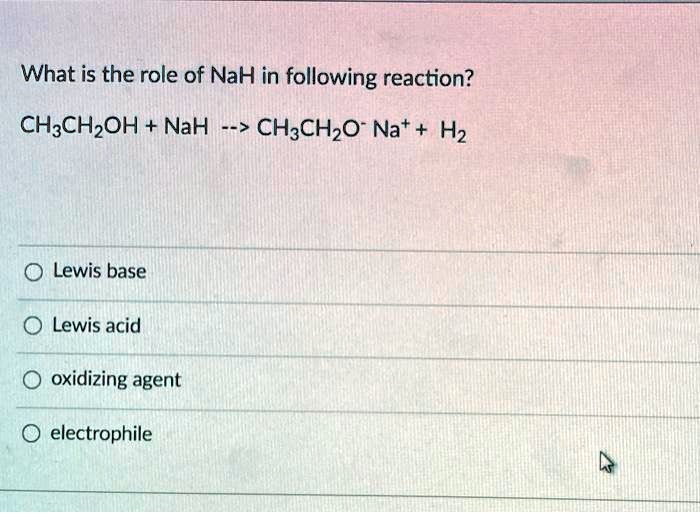
SOLVED: What is the role of NaH in following reaction? CH3CHzOH NaH 7-> CH:CHzO Nat H2 Lewis base Lewis acid oxidizing agent electrophile

Complications from dual roles of sodium hydride as a base and as a reducing agent. | Semantic Scholar

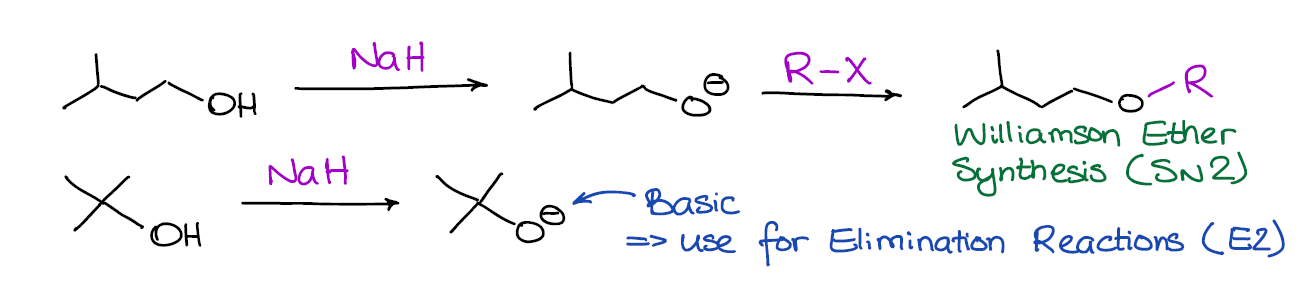
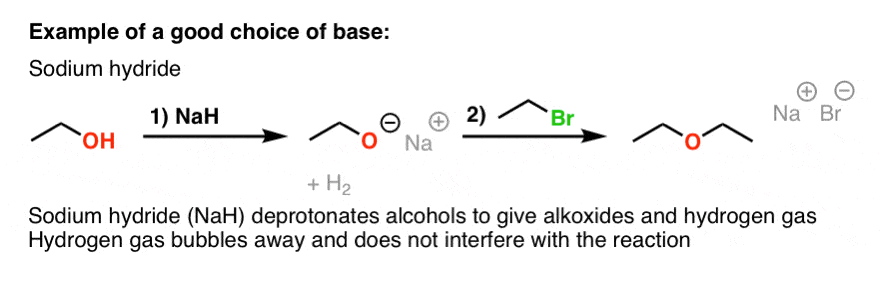
Commonly Used Hydride Reagents. Several forms of hydride (H-) find use in organic chemistry, including NaH, CaH 2, LiAlH 4, NaBH 4, and NaBH 3 CN (and. - ppt download

The hydride ion ( H^ - ) is stronger base than OH^ - ion. Which of the following reaction will occurs if sodium hydride (NaH) is dissolved in water?

Complications from Dual Roles of Sodium Hydride as a Base and as a Reducing Agent | The Journal of Organic Chemistry

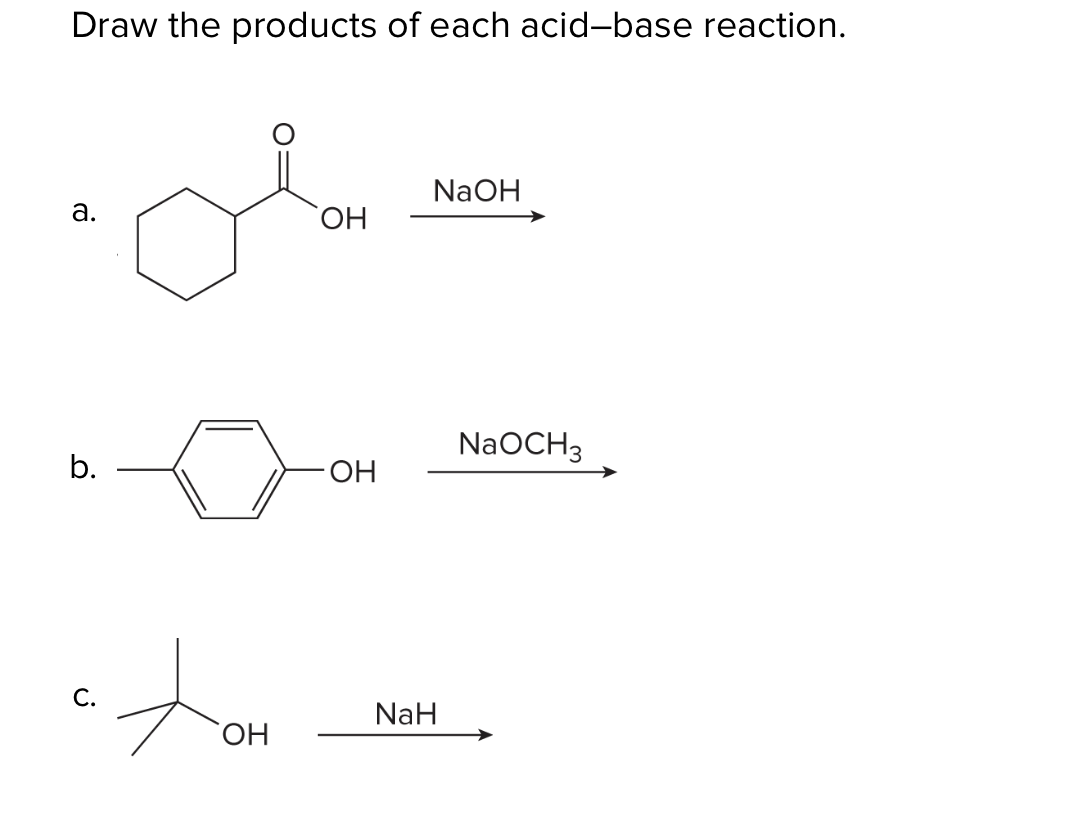
What product is formed when the given compound is treated with NaH? The given acid-base reactions were a step in a synthesis of a commercially available drug. | Homework.Study.com

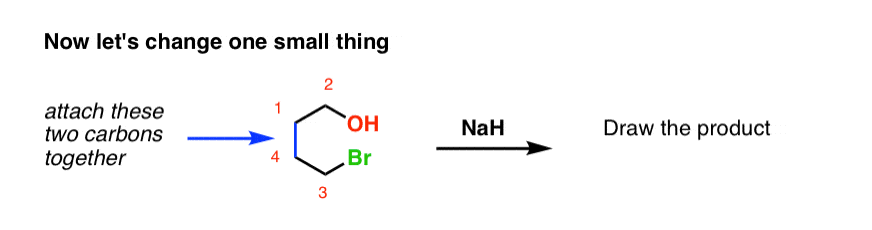
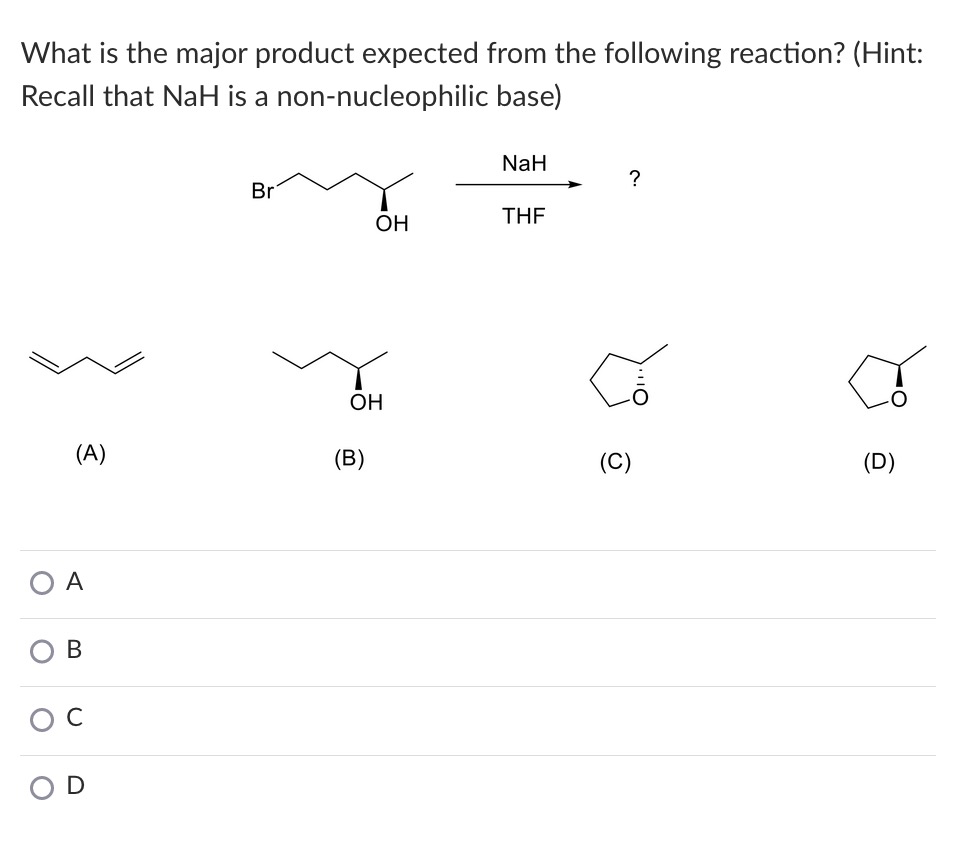
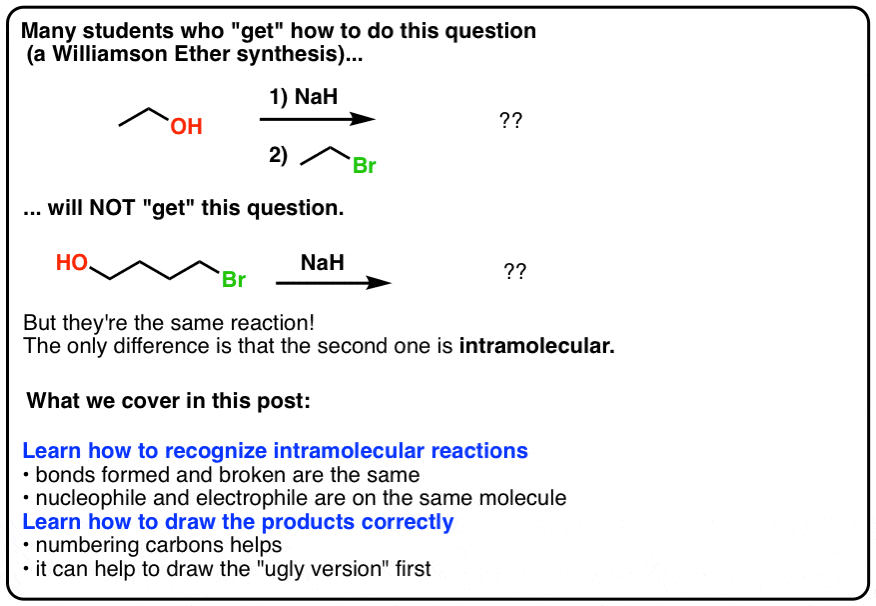
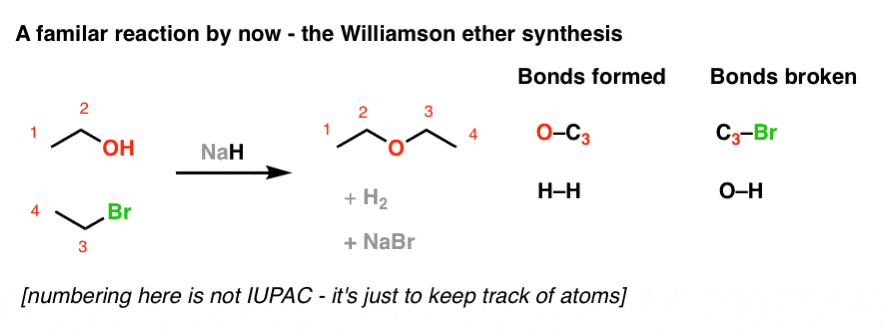
When the halohydrin is treated with NaH, a product of molecular formula C_4H_8O is formed. Draw the structure of the product and indicate its stereochemistry. | Homework.Study.com
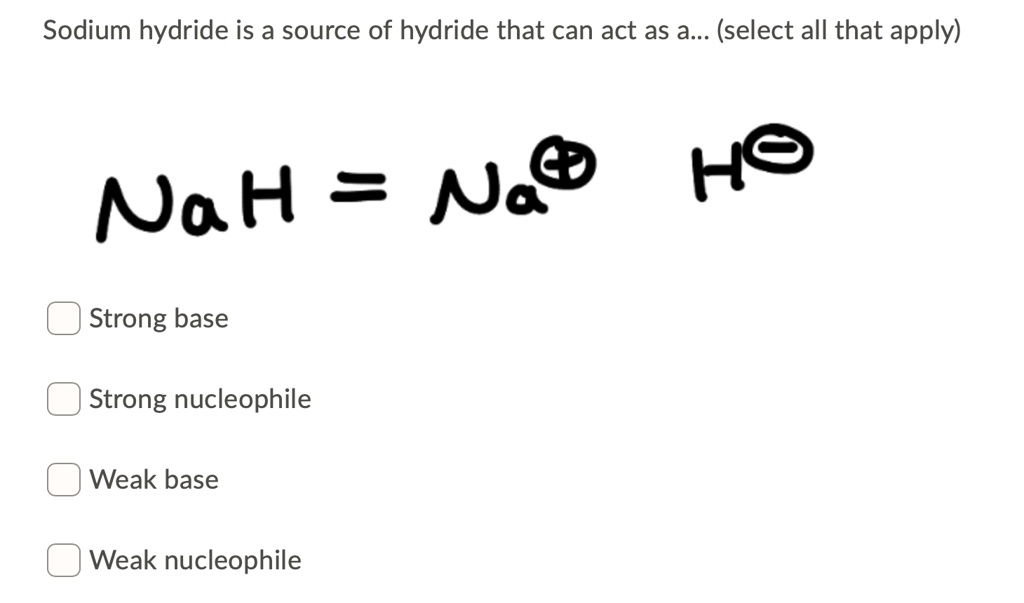
SOLVED: Sodium hydride is a source of hydride that can act as a (select all that apply) Na@ HO NaH = Strong base Strong nucleophile Weak base Weak nucleophile

Which of the following acid-base reactions will occur? f. H-C-=CNa+CH3OH g. H-C-=CH+CH3Li h. H-C-=CH+NaH i. H-C-=CH+NaCN j. H-C-=CNa+CH3COOH