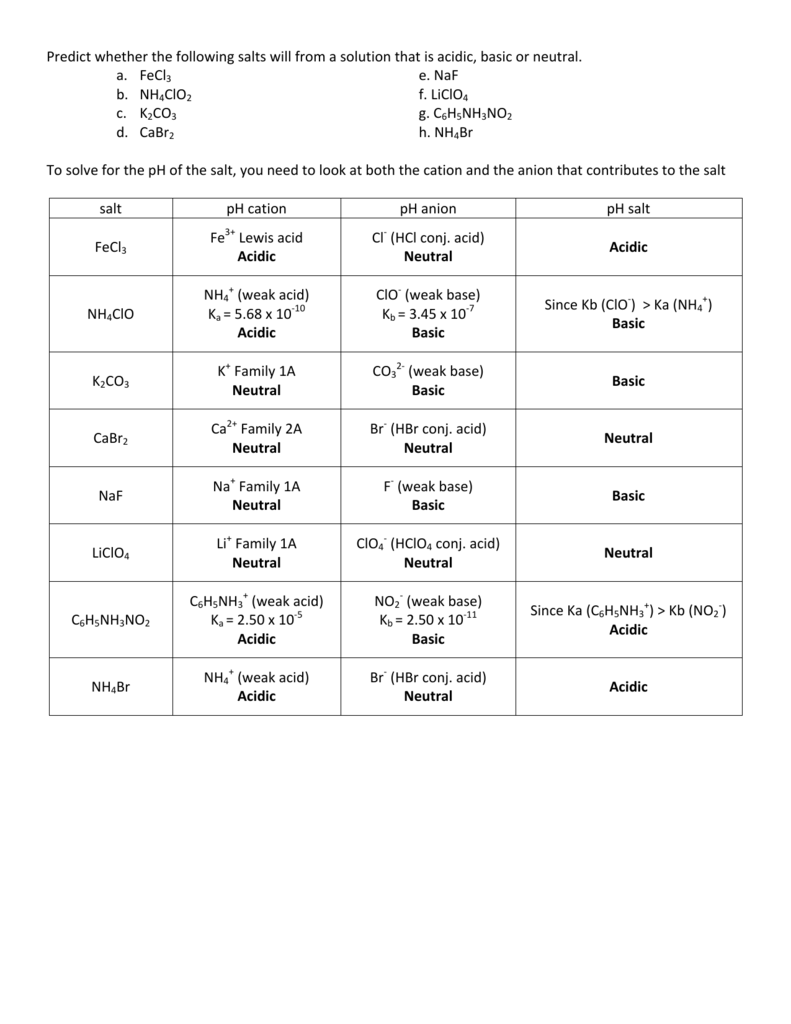
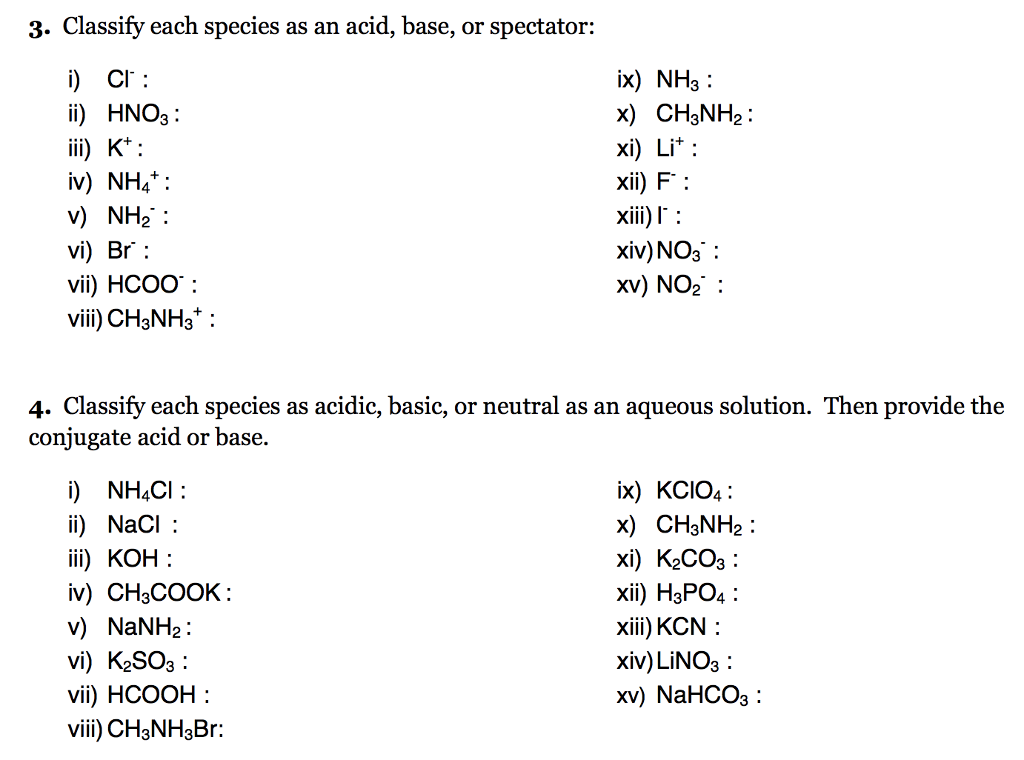
Classify these salts as acidic, basic, or neutral. And Why? NH4ClO4 , KCl , LiNO3 , NaCN , K2CO3 - YouTube
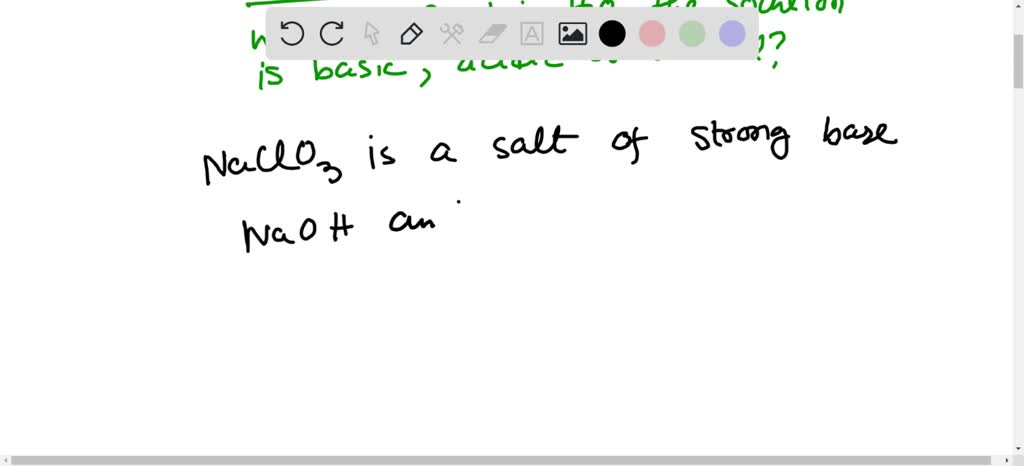
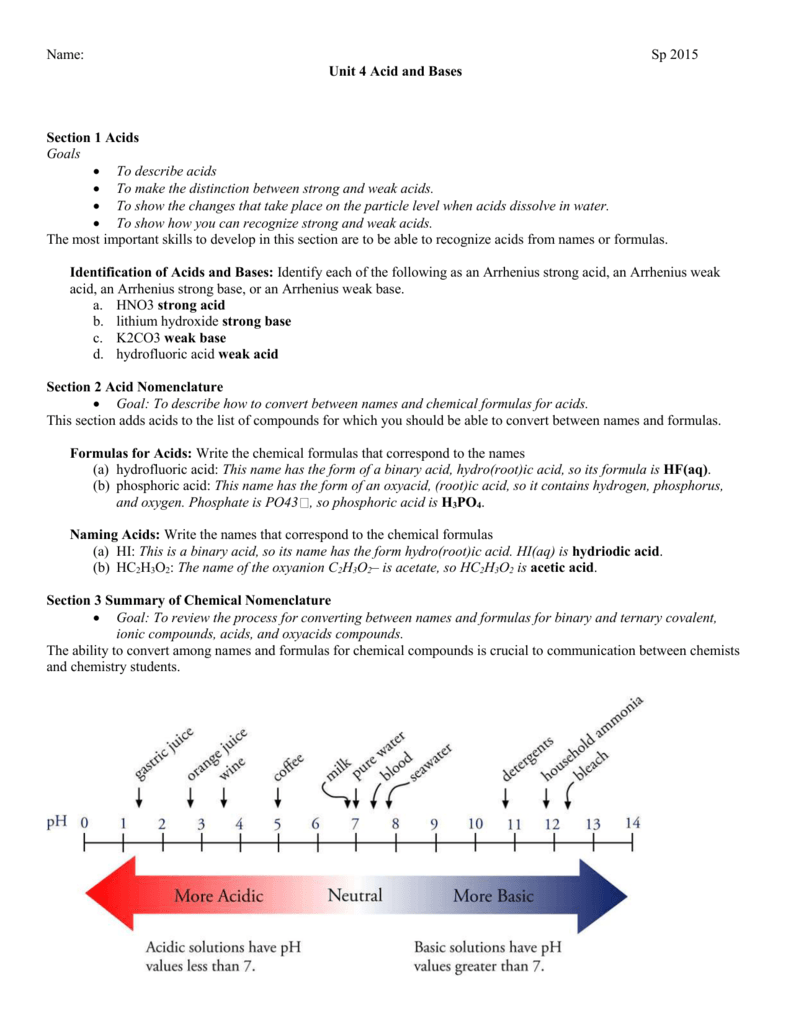
SOLVED: When K2CO3 is dissolved in water, will the solution be acidic, basic, neutral, or will more information be needed to decide?
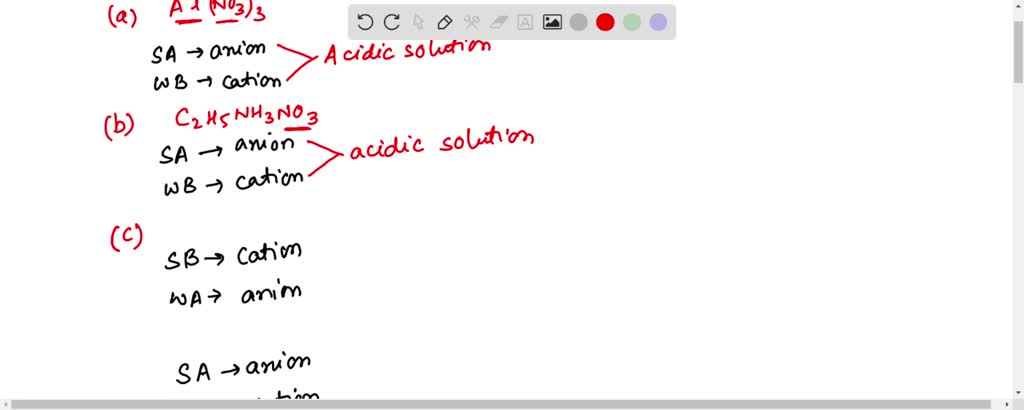
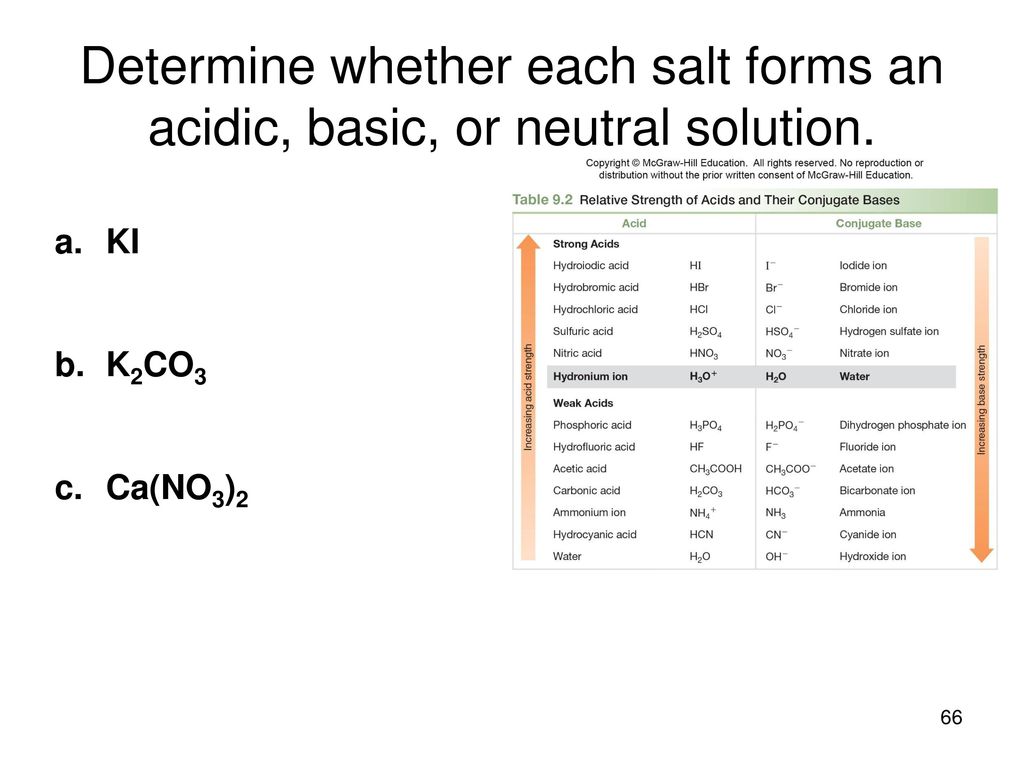
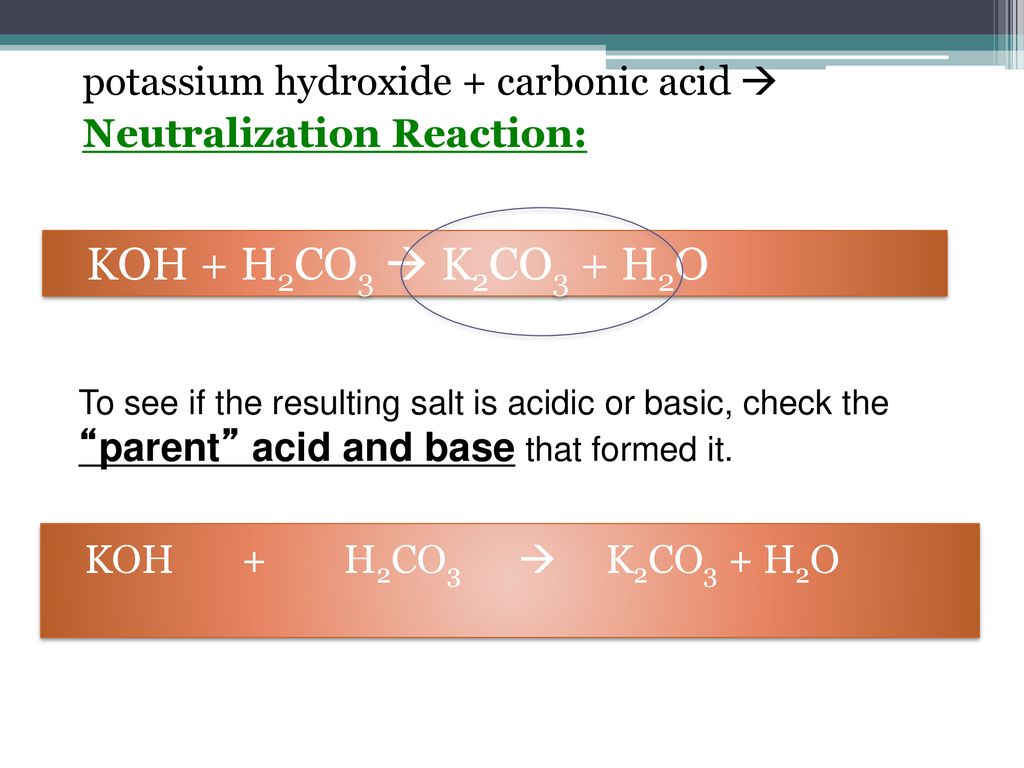
SOLVED: Determine if each salt will form a solution that is acidic, basic, or pH-neutral. a. Al(NO3)3 b. C2H5NH3NO3 c. K2CO3 d. RbI e. NH4ClO