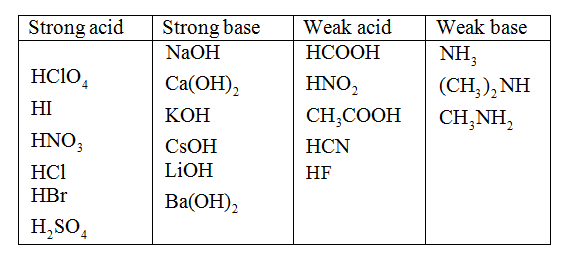
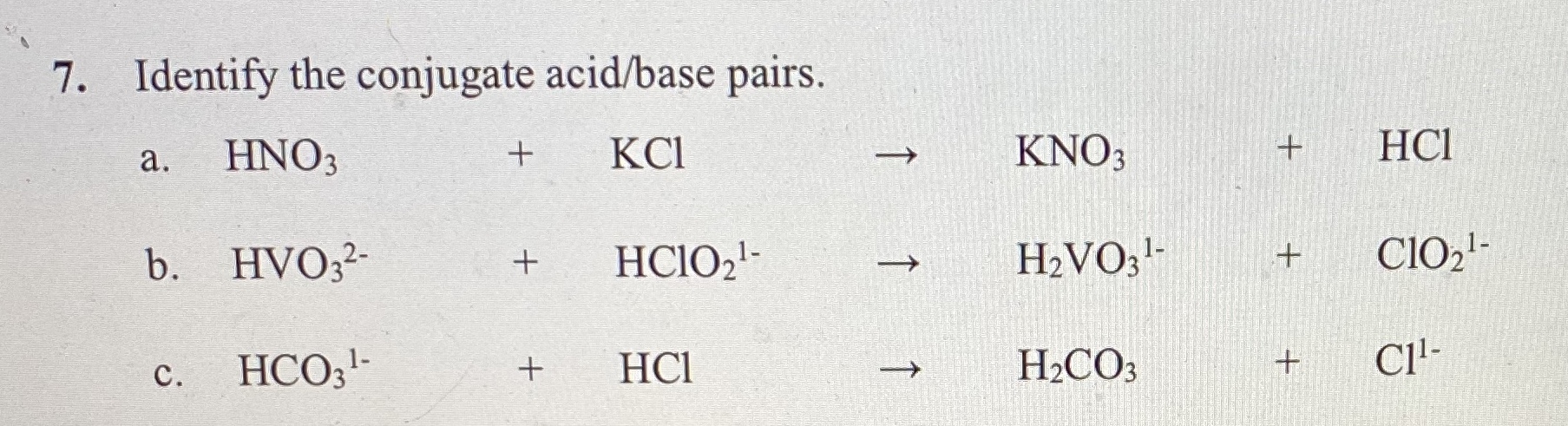
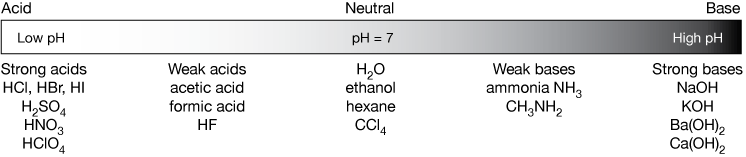
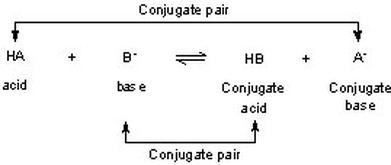
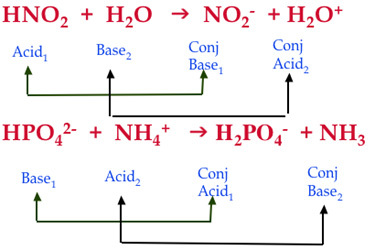
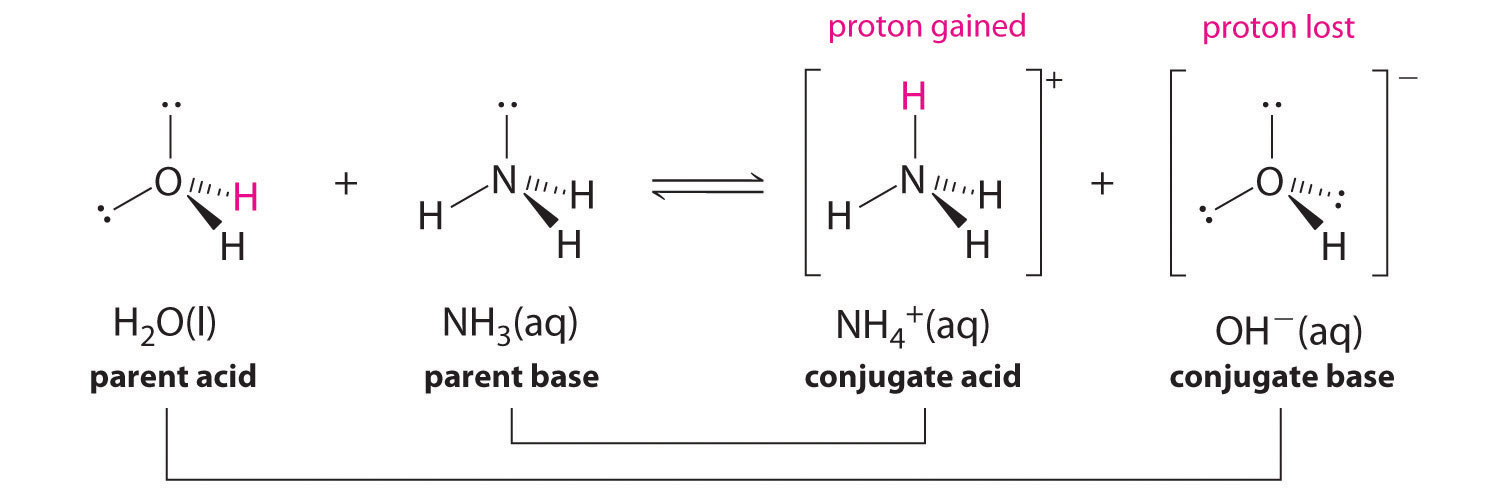
inorganic chemistry - Comparison of acidic strength between nitric acid and nitrous acid - Chemistry Stack Exchange
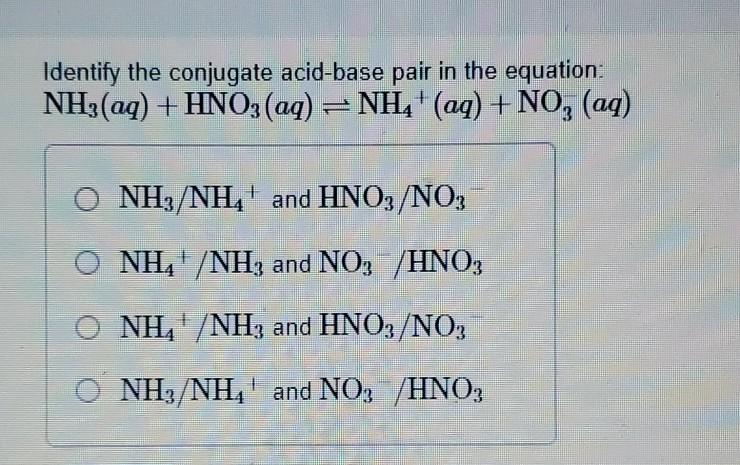
Sort out the conjugate pairs of acid and bases in the following reactions : HNO3(aq) +H2O (l)hArr H3O^(+) (aq) +NO3^(-) (aq)
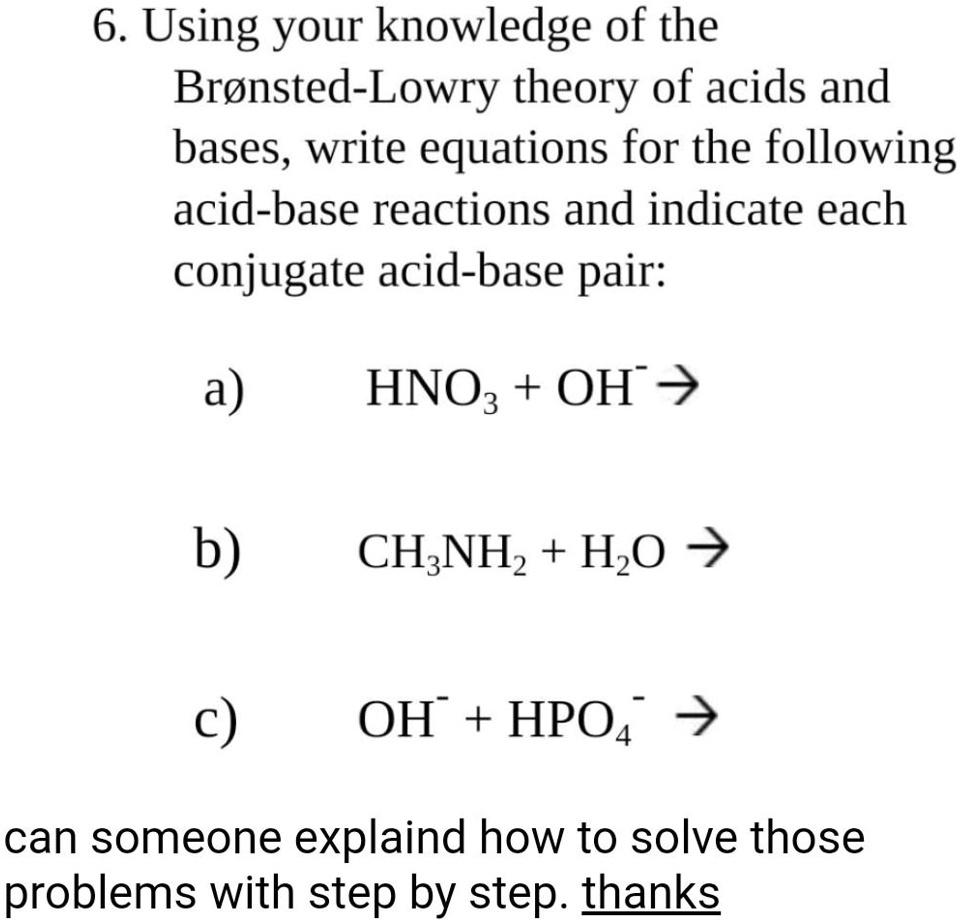
SOLVED: 6 Using your knowledge of the Bronsted-Lowry theory of acids and bases, write equations for the following acid-base reactions and indicate each conjugate acid-base pair: a) HNOz + OH b) CH;NHz +
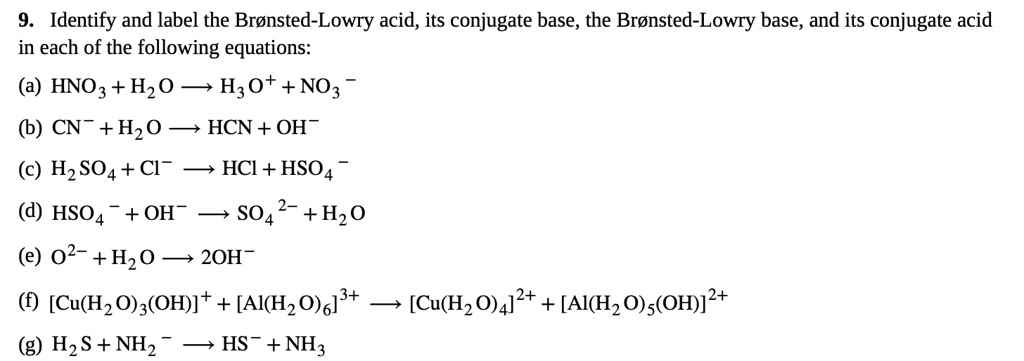
SOLVED: Identify and label the Bronsted-Lowry acid, its conjugate base, the Bronsted-Lowry base, and its conjugate acid in each of the following equations: (a) HNO3 + Hz0 HzO++ NO3 (6) CN: +Hz0