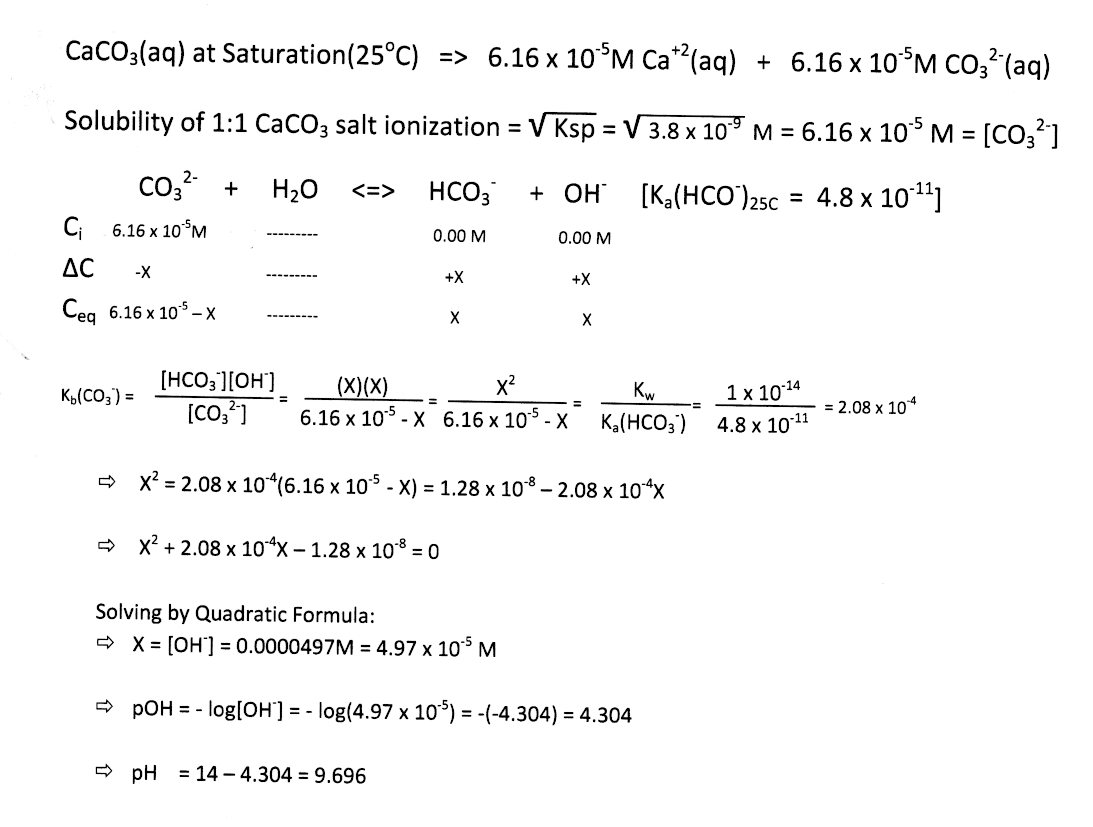
Question Video: Calculating the Mass of Calcium Carbonate Required to Produce a Given Mass of Calcium Oxide | Nagwa
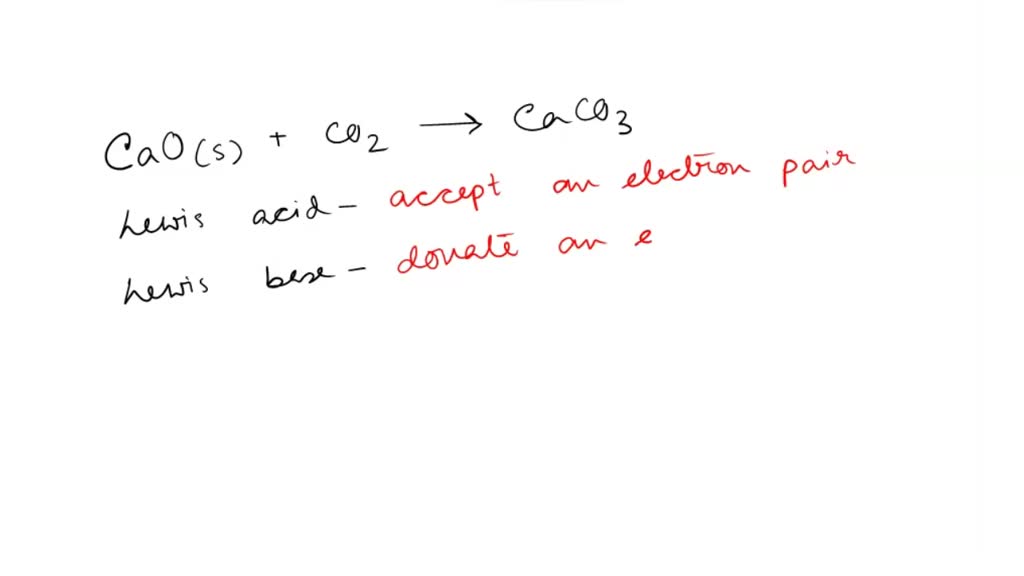
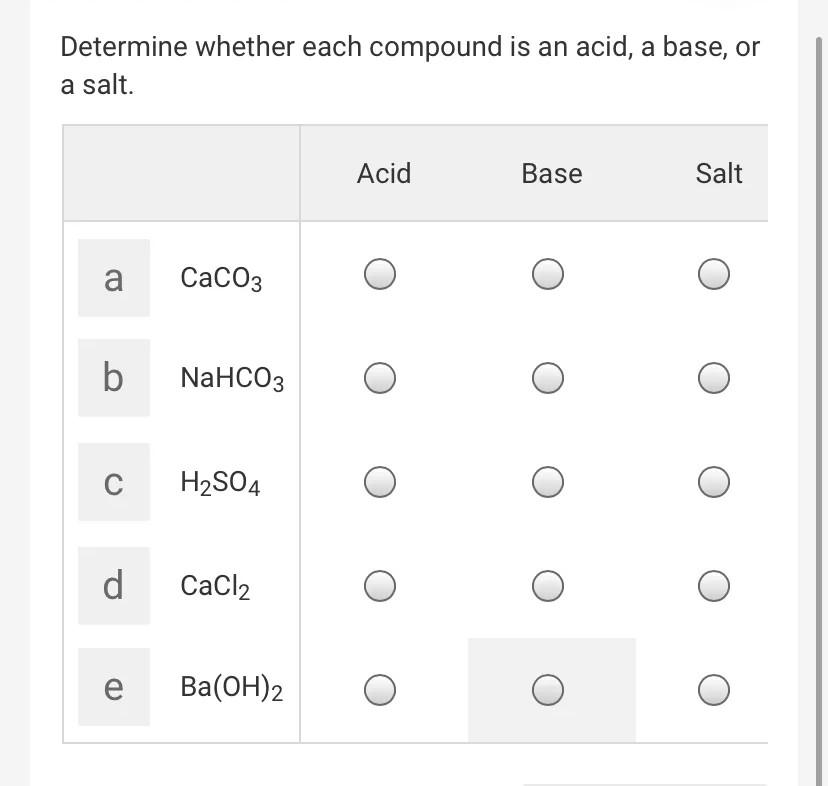
SOLVED: 'QUESTION 1 In the reaction: CaO(s) + C02(g) 5 CaCO3(s) 0A Ca2+acts as a Lewis acid and CO32- acts as a Lewis base 02-acts as a Lewis base and CO2 acts

Everyday acid and base reactions. Calcium carbonate and rocks. Limestone is also largely composed of calcium carbonate. Bath Stone (Greater Oolite) is. - ppt download

CBSE Class 10 science term 1 Question Solution | parent acid and base of Calcium carbonate is....... - YouTube
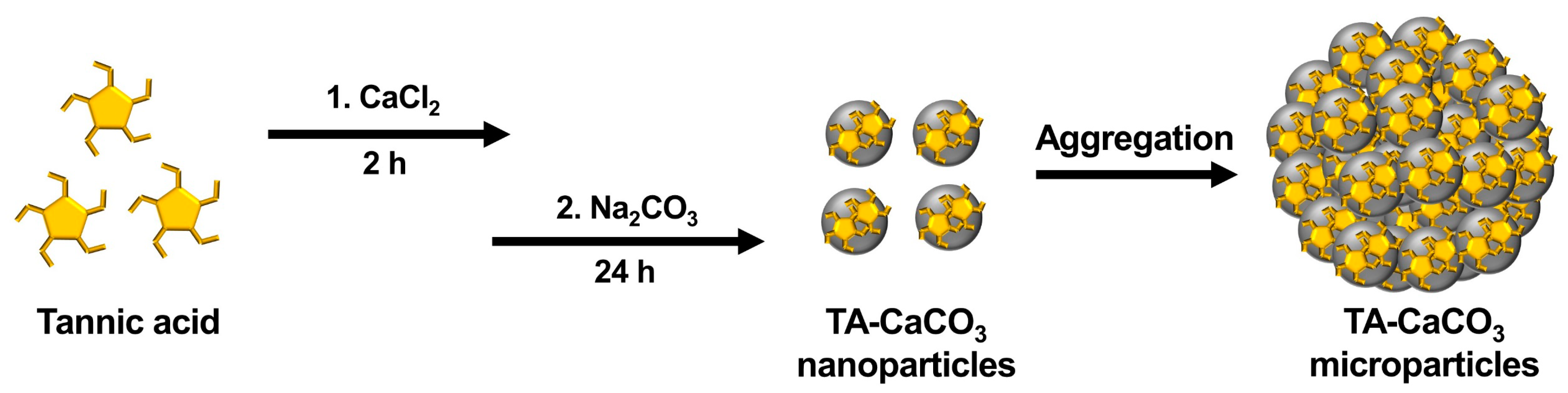
IJMS | Free Full-Text | Tannylated Calcium Carbonate Materials with Antacid, Anti-Inflammatory, and Antioxidant Effects
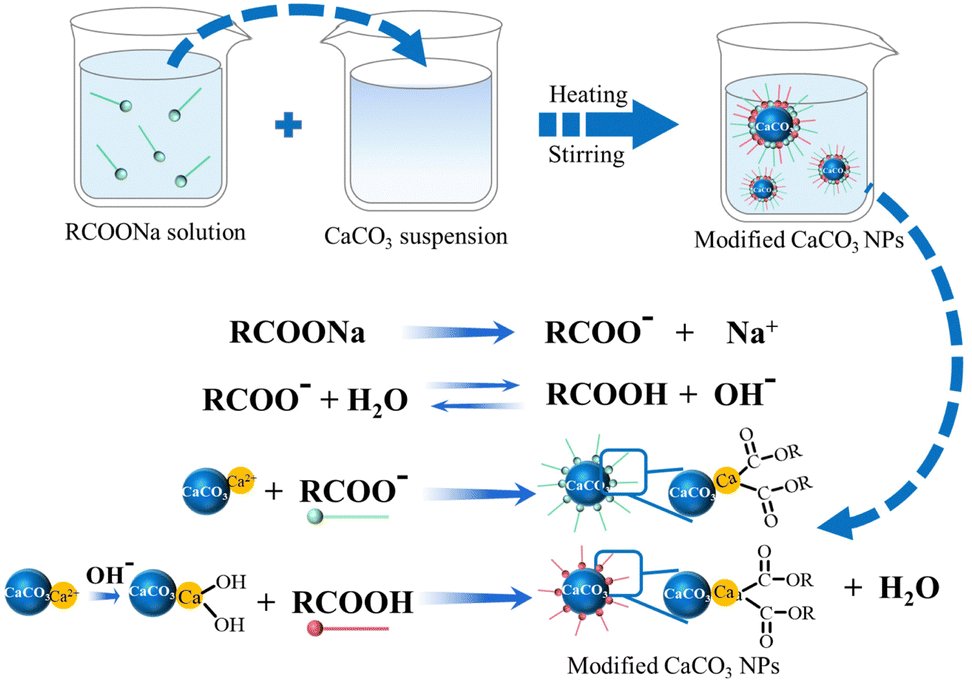
Calcium carbonate: controlled synthesis, surface functionalization, and nanostructured materials - Chemical Society Reviews (RSC Publishing) DOI:10.1039/D1CS00519G

Acid-base reaction of naproxen and calcium carbonate, containing the... | Download Scientific Diagram






![MCQ] - Which correctly represents Parent acid and base of Calcium MCQ] - Which correctly represents Parent acid and base of Calcium](https://d1avenlh0i1xmr.cloudfront.net/475685cc-4d58-4e74-a739-8cbc5d1c10f8/q8---parent-acid-and-base-of-calcium-carbonate---teachoo.jpg)


![MCQ] - Which correctly represents Parent acid and base of Calcium MCQ] - Which correctly represents Parent acid and base of Calcium](https://d1avenlh0i1xmr.cloudfront.net/0014c0c3-c848-4073-a814-6e71c0e2bf5e/reaction-to-form-calcium-carbonate---teachoo-01.jpg)