Trimyristin can be hydrolyzed in base NaOH to form myristic acid. Write a balanced chemical reaction. Trimyristin should be shown in line/bond form. | Homework.Study.com

Selective Focus of Sodium Hydroxide Base and Sulfuric Acid Solution in Brown Glass and Plastic Bottle Stock Image - Image of harmful, class: 195465085
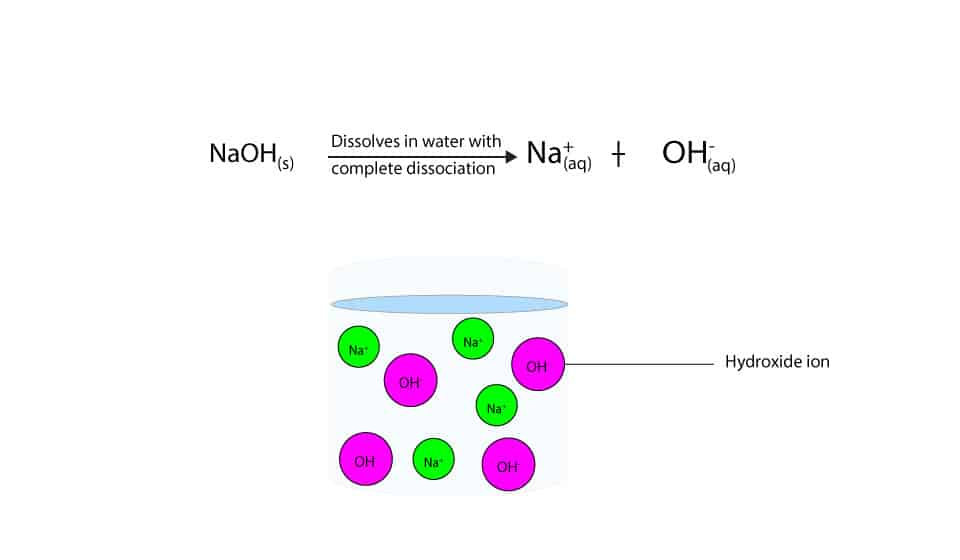
Sodium hydroxide (NaOH) is classified as a strong base. For every mole of sodium hydroxide added to a large volume of water, one mole of what ion enters the solution? | Socratic
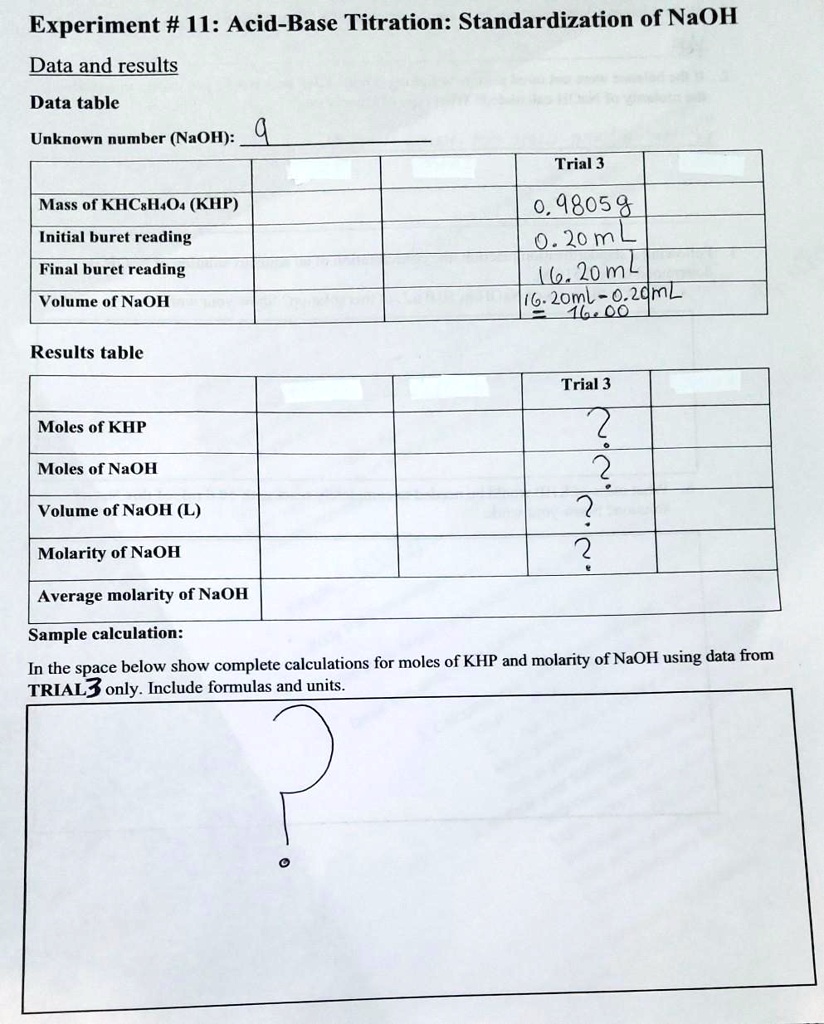
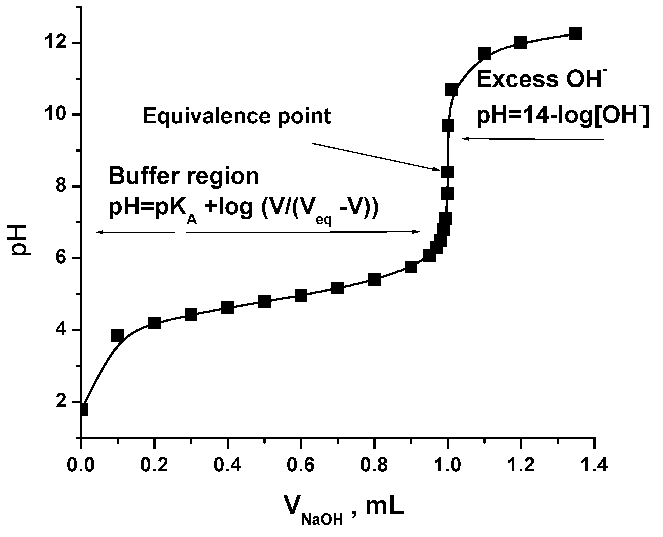
SOLVED: Experiment # 1: Acid-Base Titration: Standardization of NaOH Data and results Data table Unknown number (NaOH): Trial 3 Mass of KHC lO4 (KHP) 4805.9 0.20m 6.2o ml (6. zon 24L 0O
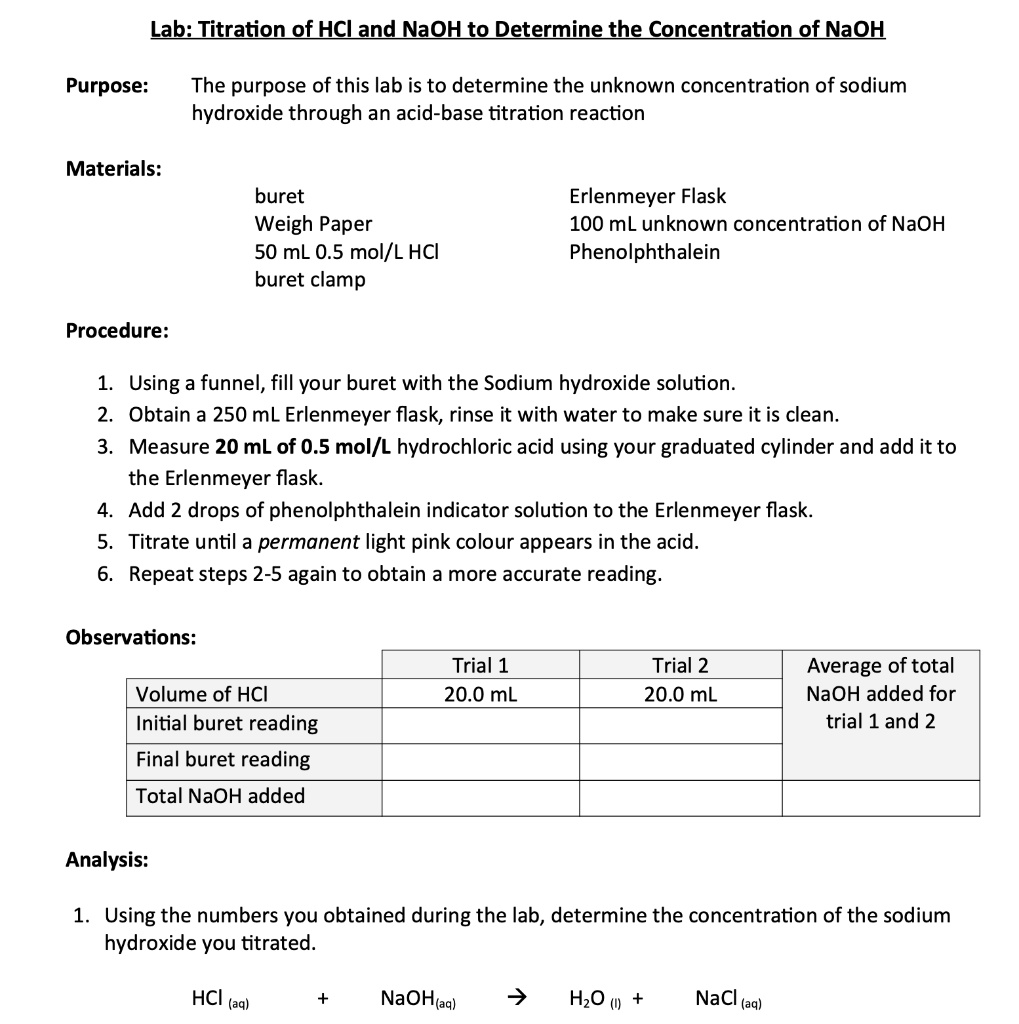
SOLVED: Lab: Titration of HCLand NaOHto Determine the Concentration of NaOH Purpose: The purpose of this lab is to determine the unknown concentration of sodium hydroxide through an acid-base titration reaction Materials:
Sodium hydroxide, caustic soda, lye molecule. NaOH is highly caustic base and alkali, ionic compound. Structural chemical formula and molecule model Stock Vector Image & Art - Alamy

Vector Illustration Of Electrolytic Dissociation Molecules Break Up Into Ions Chemical Containers With Acid Base And Salt Hcl Naoh And Nacl Stock Illustration - Download Image Now - iStock
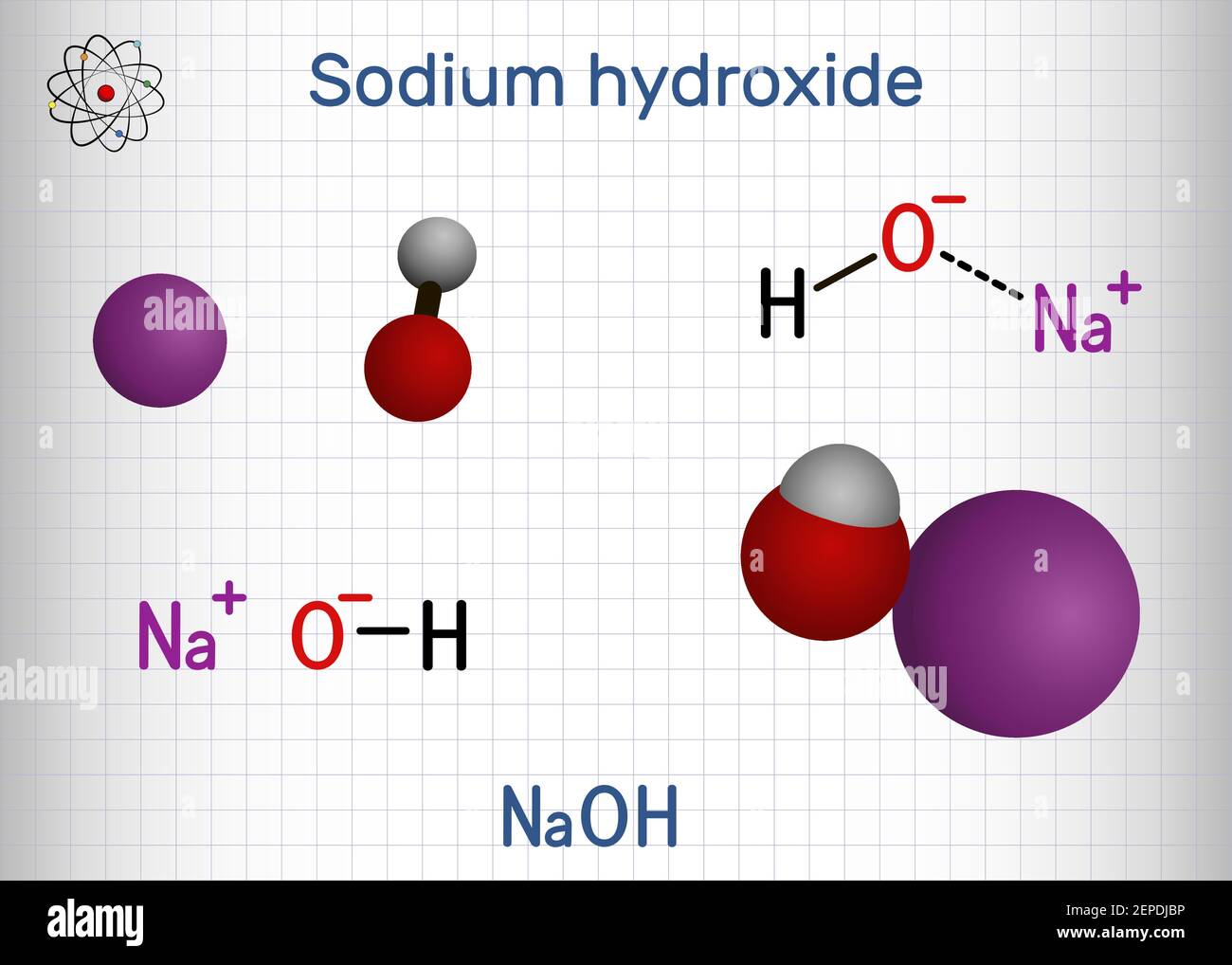
Sodium hydroxide, caustic soda, lye molecule. NaOH is highly caustic base and alkali, ionic compound. Structural chemical formula and molecule model Stock Vector Image & Art - Alamy

RICCA CHEMICAL COMPANY - Sodium Hydroxide is a strong base in terms of chemical ionization and solutions of it can be assayed using a strong acid, such as Hydrochloric Acid or Sulfuric






-in-water-01.jpg)