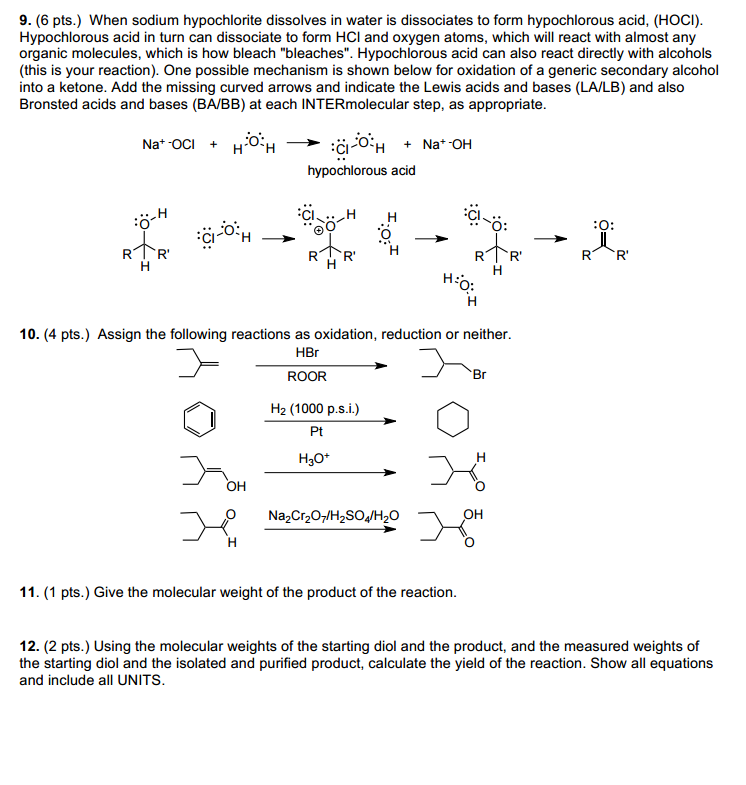
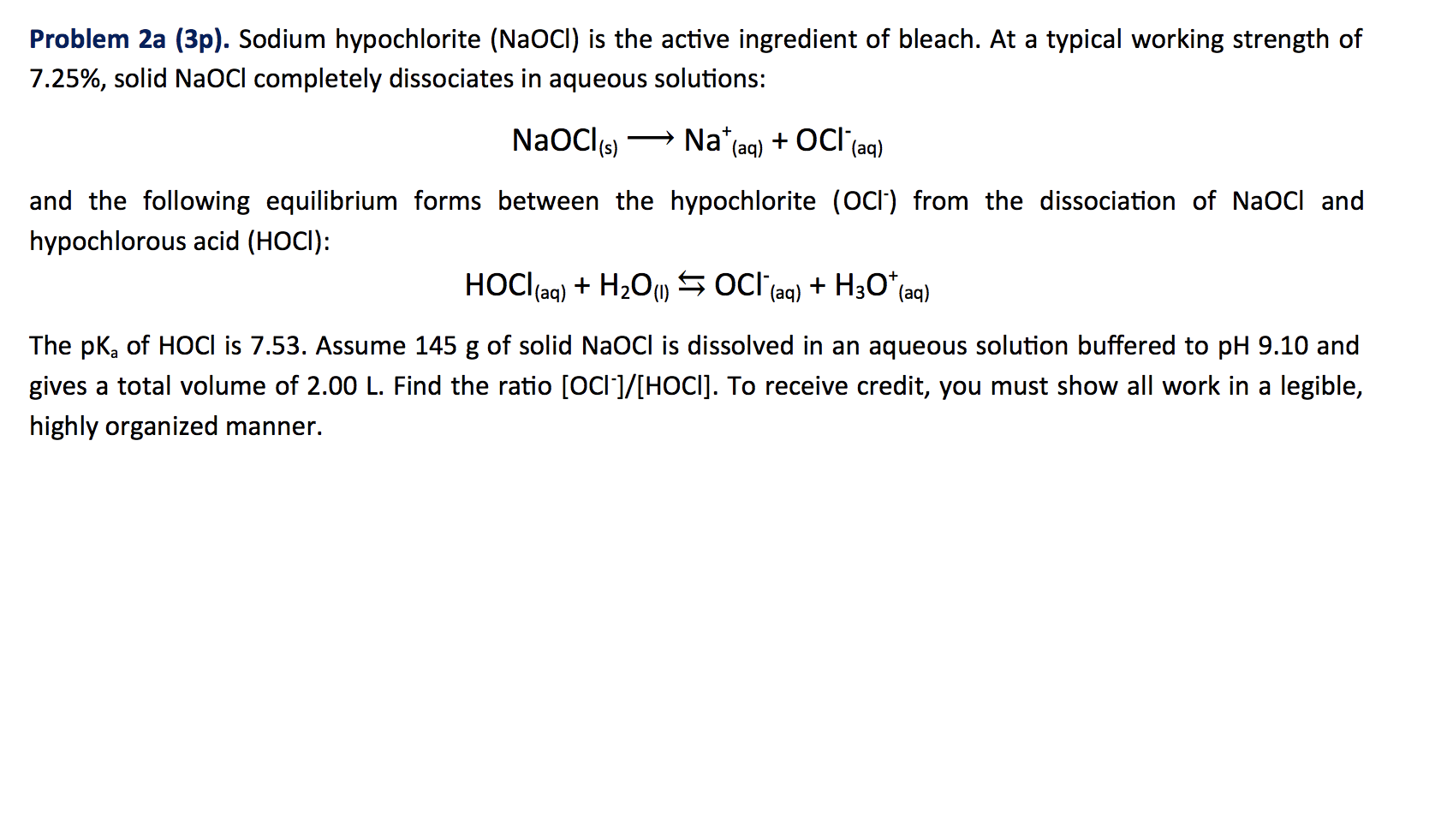
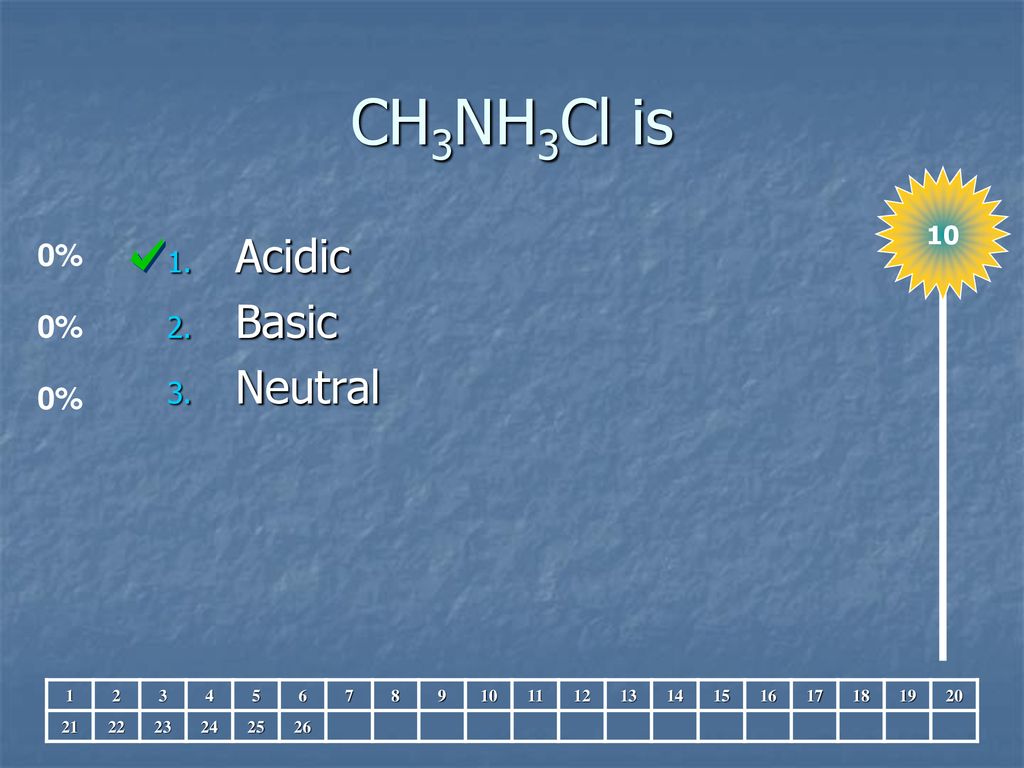
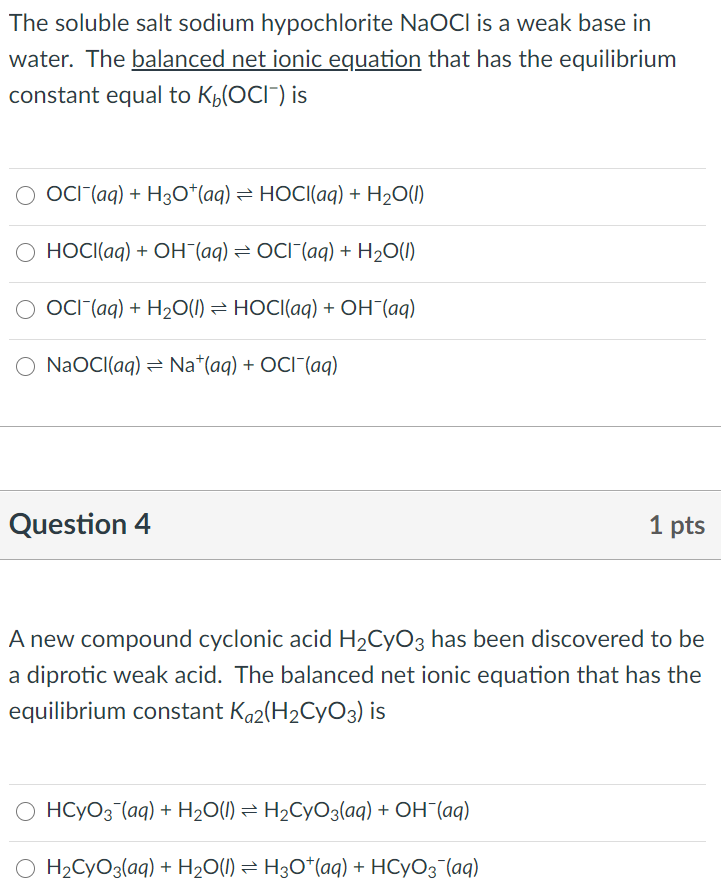
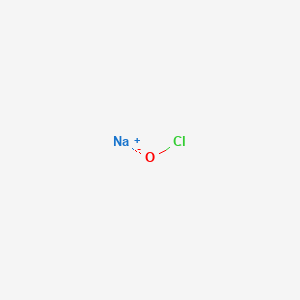
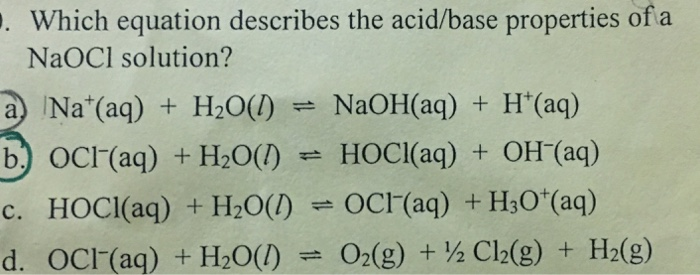SOLVED: Match the following salts with the acid-base neutralization reactants that would produce them: NaCIO4 A HCI + Ca(OH)z NaOCl B. HOCI + Ca(OH)z Ca(OCl)z C.HCI + NaOH D.HCIO4 Ca(OH)2 E: HCIO4 +

Difference Between Sodium Hypochlorite and Hypochlorous Acid | Compare the Difference Between Similar Terms

Difference Between Sodium Hypochlorite and Hypochlorous Acid | Compare the Difference Between Similar Terms

Sodium Hypochlorite Pentahydrate Crystals (NaOCl·5H2O): A Convenient and Environmentally Benign Oxidant for Organic Synthesis | Organic Process Research & Development

Acid And Base - Sodium Hypochlorite Solution (NaOCl) , Cas No- 7681-52-9 Authorized Wholesale Dealer from Kolkata

![Is NaOCl Acidic or Basic [Acids and Bases] - YouTube Is NaOCl Acidic or Basic [Acids and Bases] - YouTube](https://i.ytimg.com/vi/HXJWALr3BEY/maxresdefault.jpg)