Acid–base titration curves of the biomass pretreated with NaHCO3 sat.... | Download Scientific Diagram

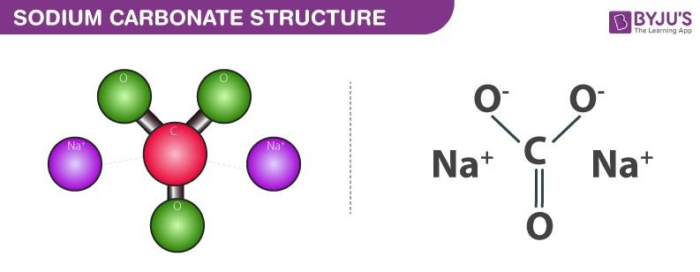
Write a mechanism (using curved-arrow notation) for the deprotonation of tannins in base. Use Ar-OH as a generic form of a tannin and use sodium carbonate (Na2CO3) as the base. Balance the

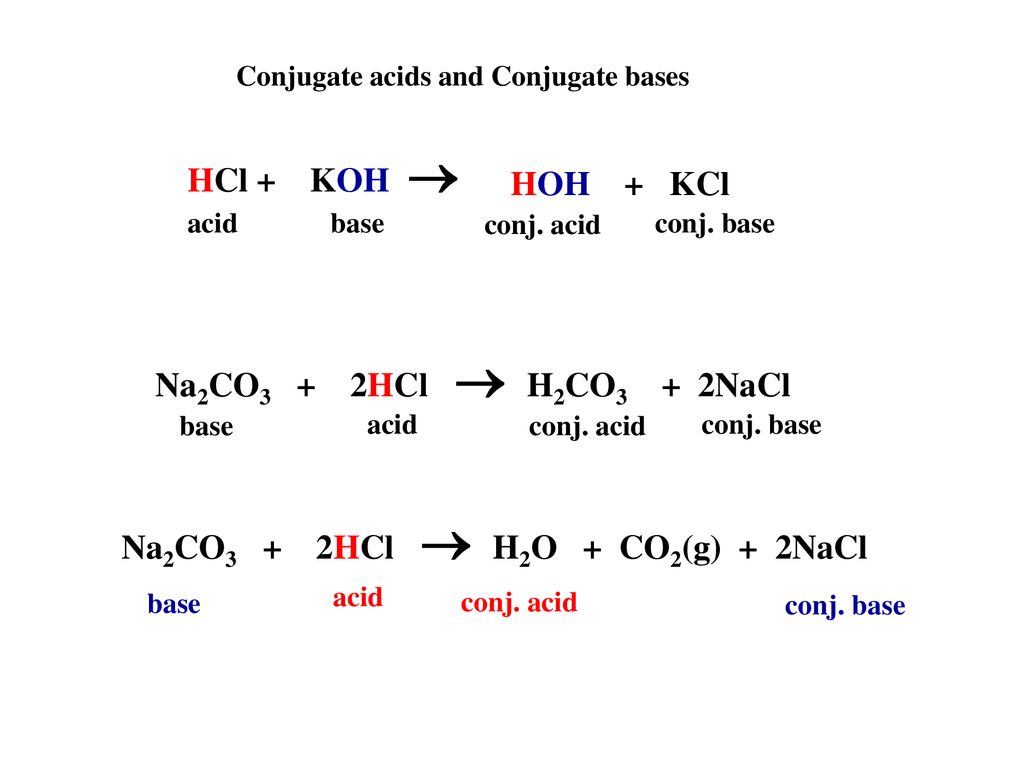
SOLVED: 1. Sodium carbonate is a primary standard base that reacts with hydrochloric acid as follows Na2CO3 + 2 HCl → 2NaCl + H2O + CO2(g) If 40.37 mL of an HCl

The titration of Na2CO3 with HCl has the following qualitative profile: a. Identify the major species in solution as points A-F. b. For the titration of 25.00 mL of 0.100 M Na2CO3
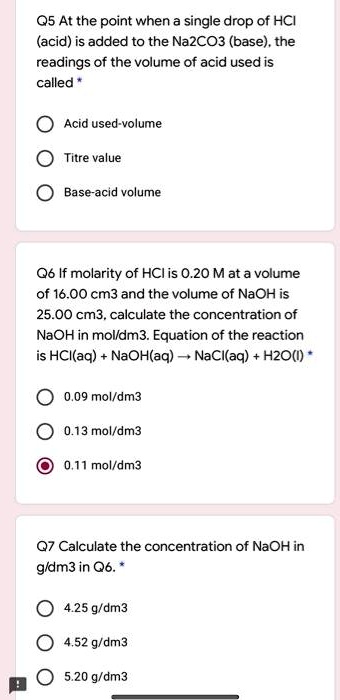
SOLVED: 05 At the point when a single drop of HCI (acid) is added to the Na2CO3 (base) . the readings of the volume of acid used is called Acid used-volume Titre

DOC) Standardization of a strong acid (HCl) with a weak base (Na2CO3).docx | Meharaj Ul Mahmmud - Academia.edu