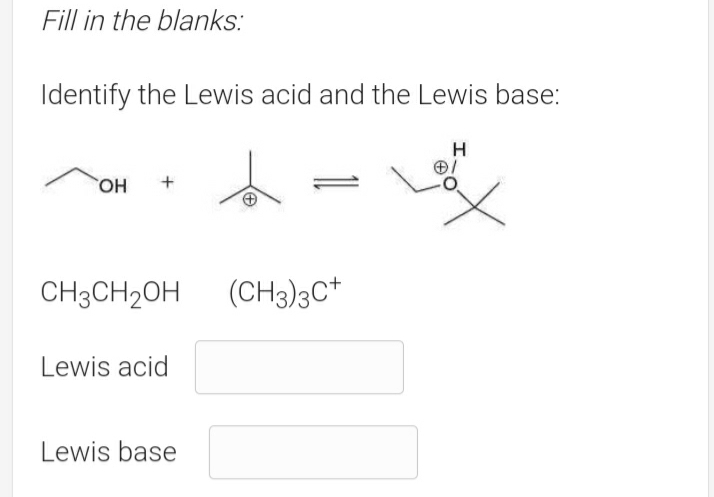
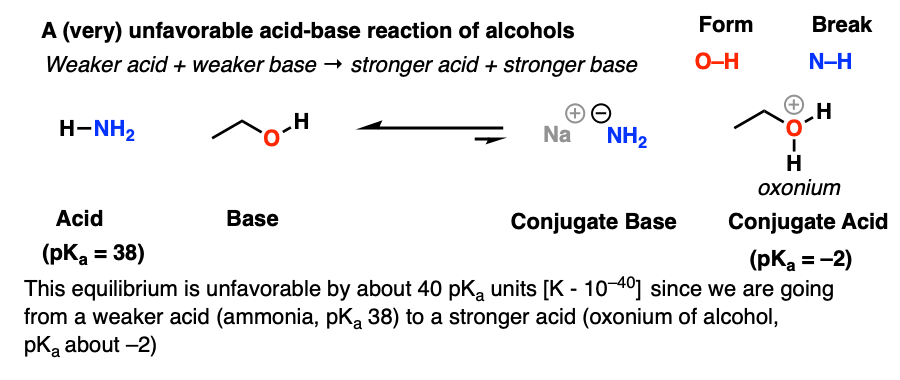Do acetic acid + ethanol produce ethyl acetate if we only mix it in a volumetric flask under room temperature? - Quora

Write a structural formula of the conjugate acid formed by the reaction of CH3CH2OH with HCl. | Homework.Study.com

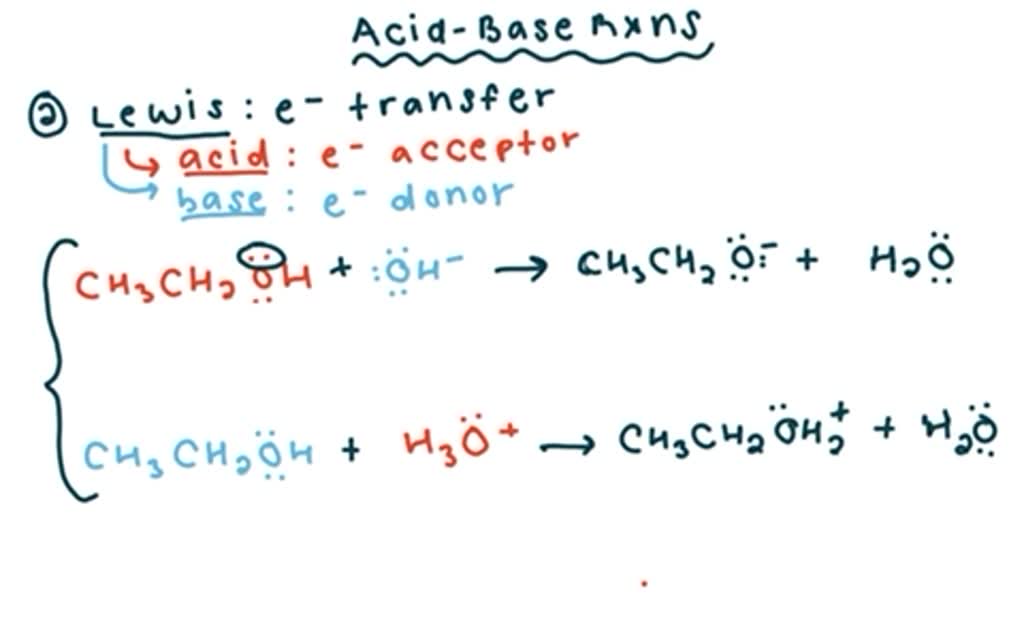
Show how the given species can act as Lewis bases in their reactions with HCl. CH_3CH_2OH, (CH_3)_2NH, (CH_3)_3P | Homework.Study.com
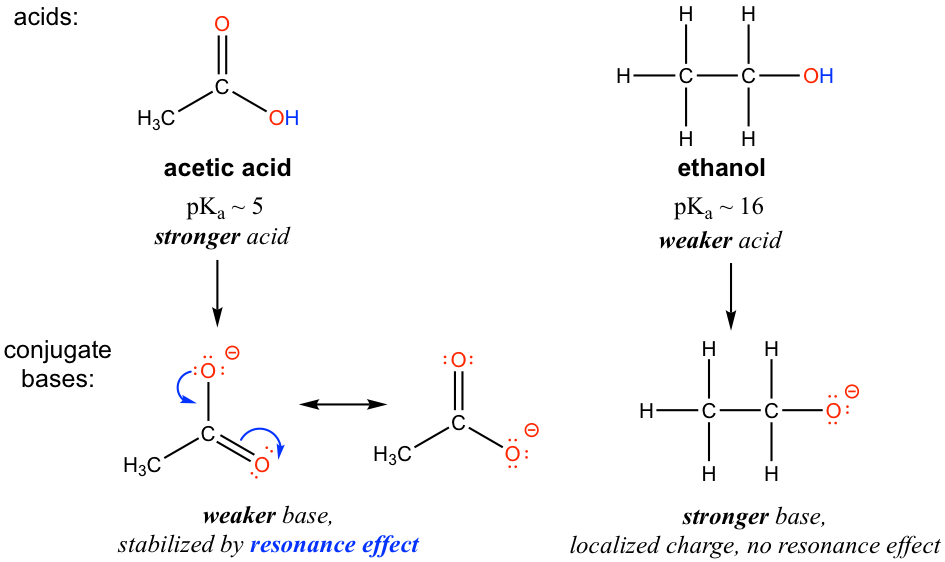
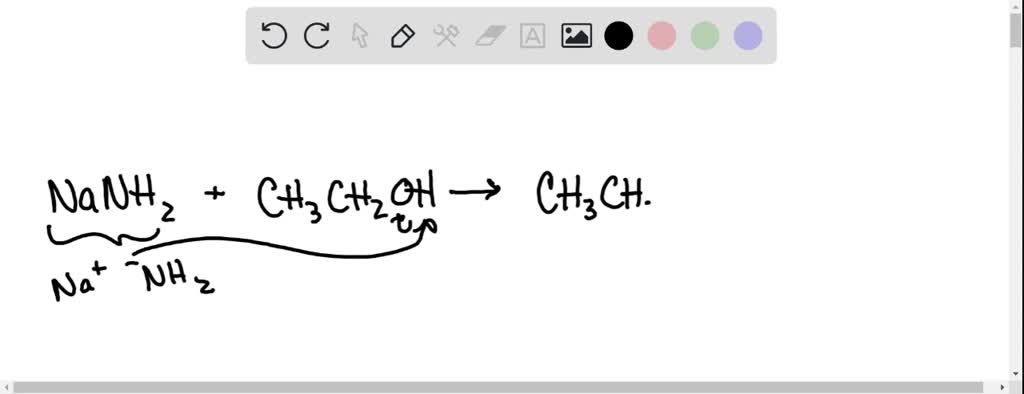
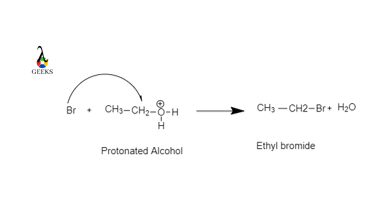


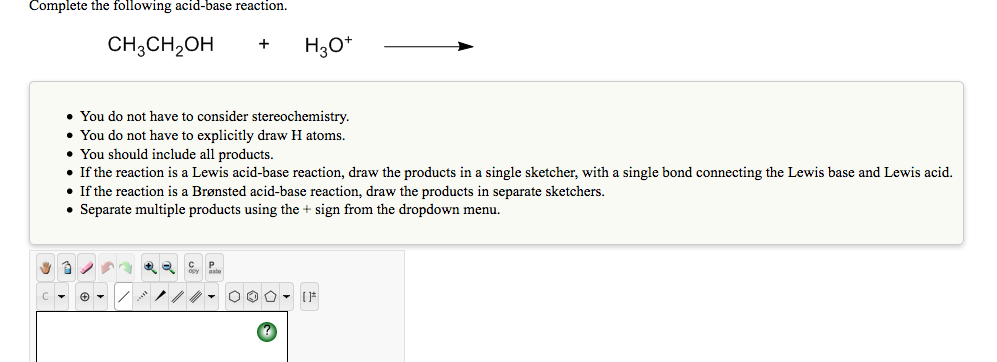

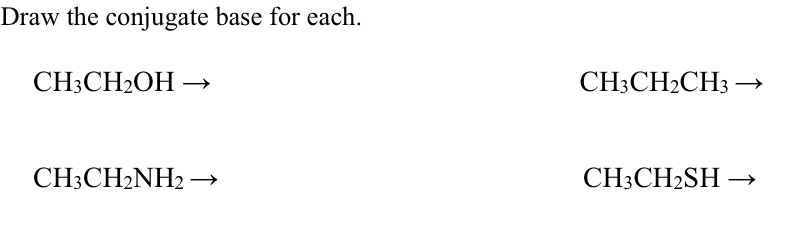
![Chemistry] Differentiate between Ethanol and Ethanoic acid - Class 10 Chemistry] Differentiate between Ethanol and Ethanoic acid - Class 10](https://d1avenlh0i1xmr.cloudfront.net/8b2659af-0499-4edb-aff1-c5a5a8beec2f/ethanol-vs-ethanoic-acid---teachoo.jpg)