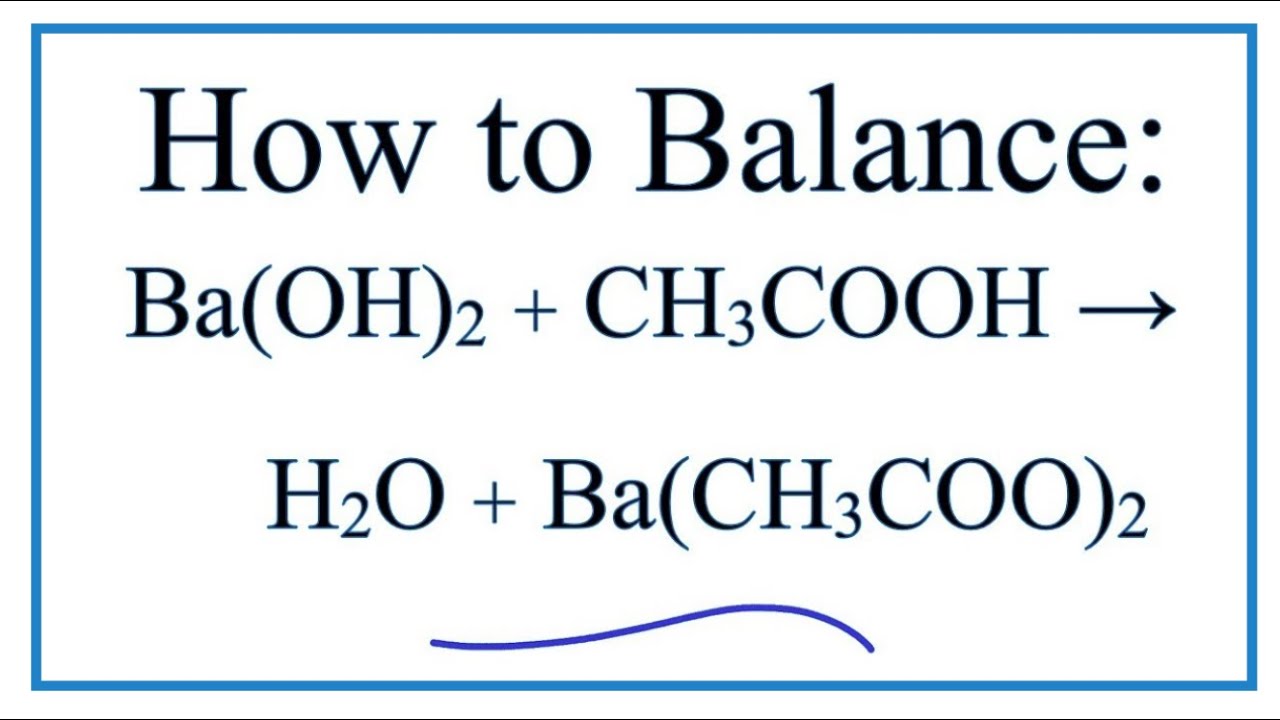
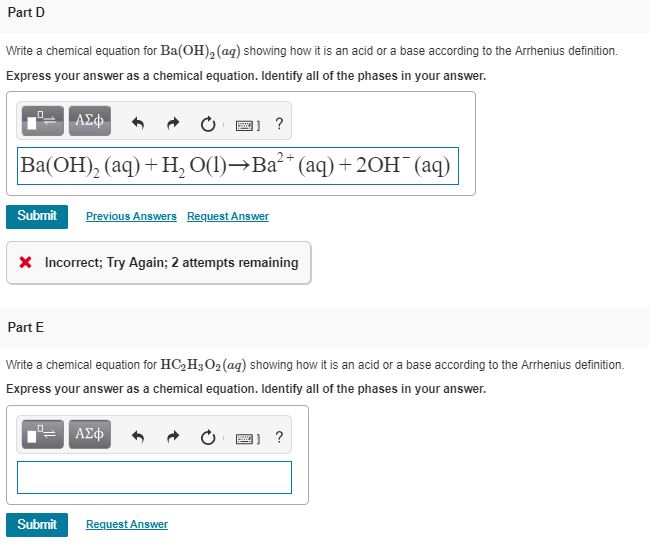
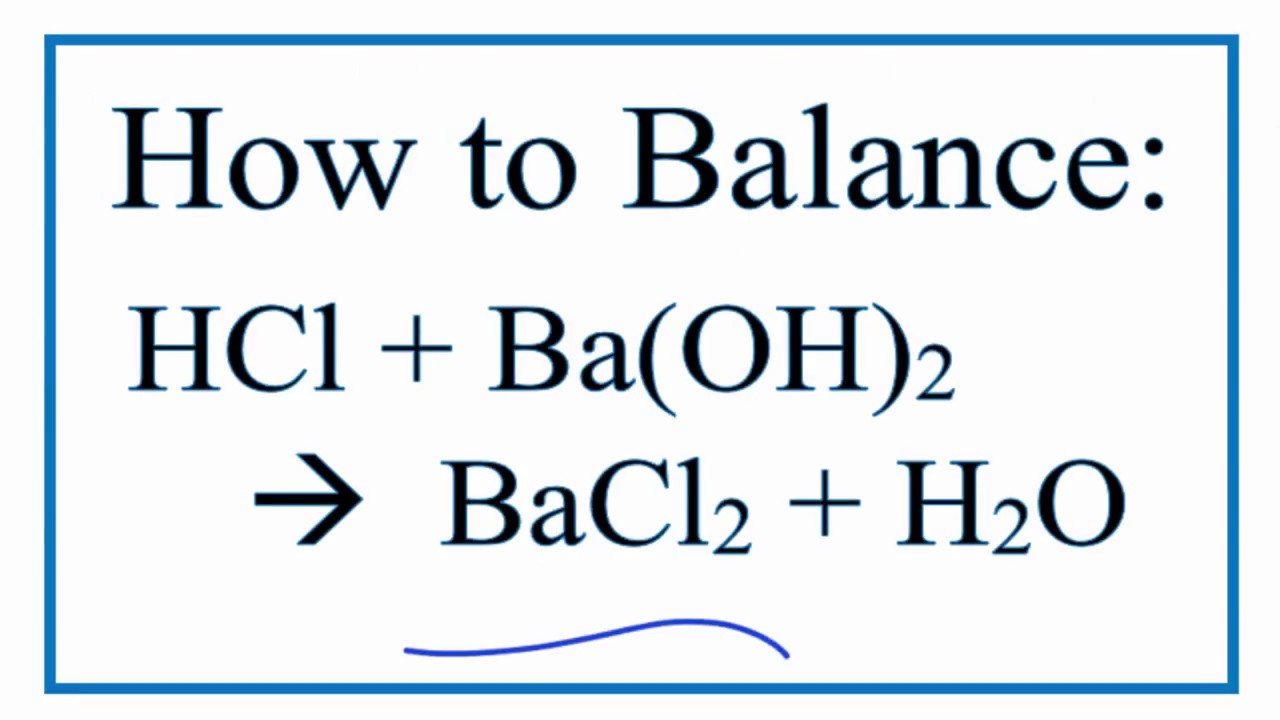
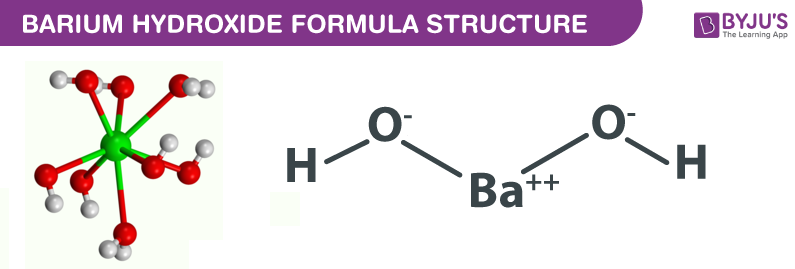
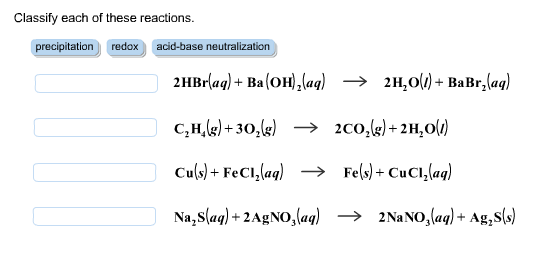
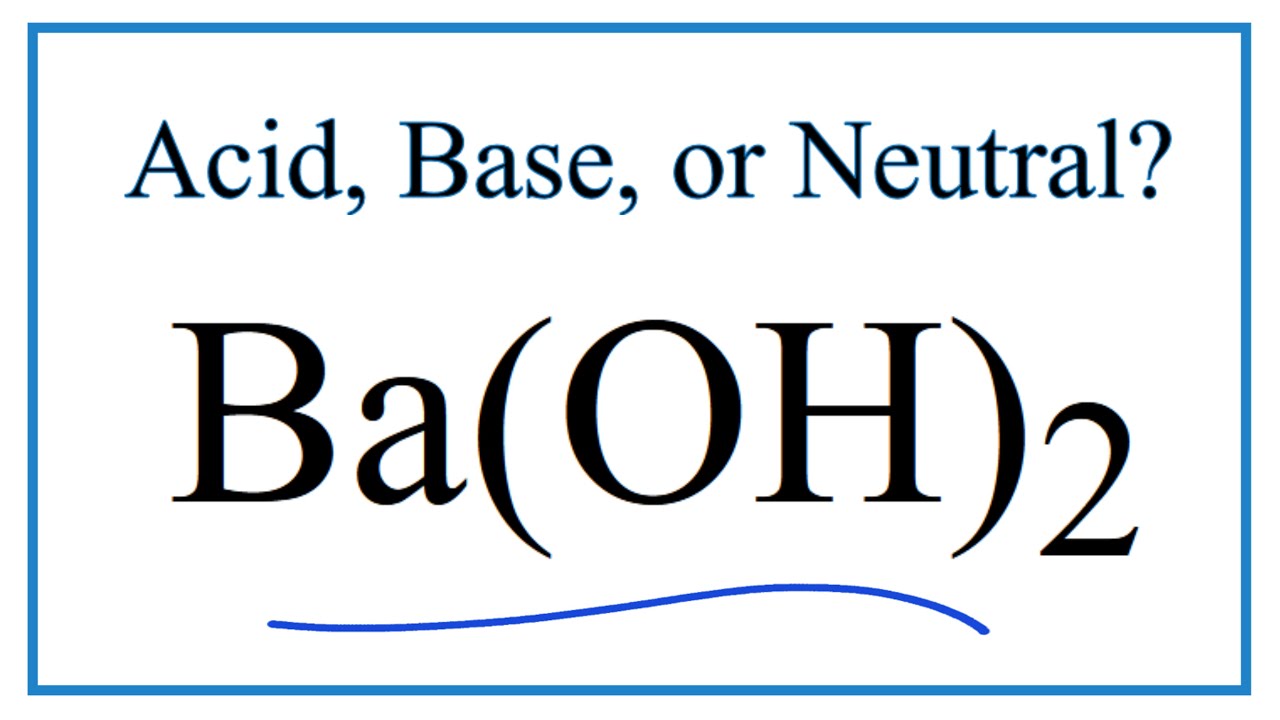A. Calculate the pH of a 0.10 M solution of barium hydroxide, Ba(OH)2 - Home Work Help - Learn CBSE Forum

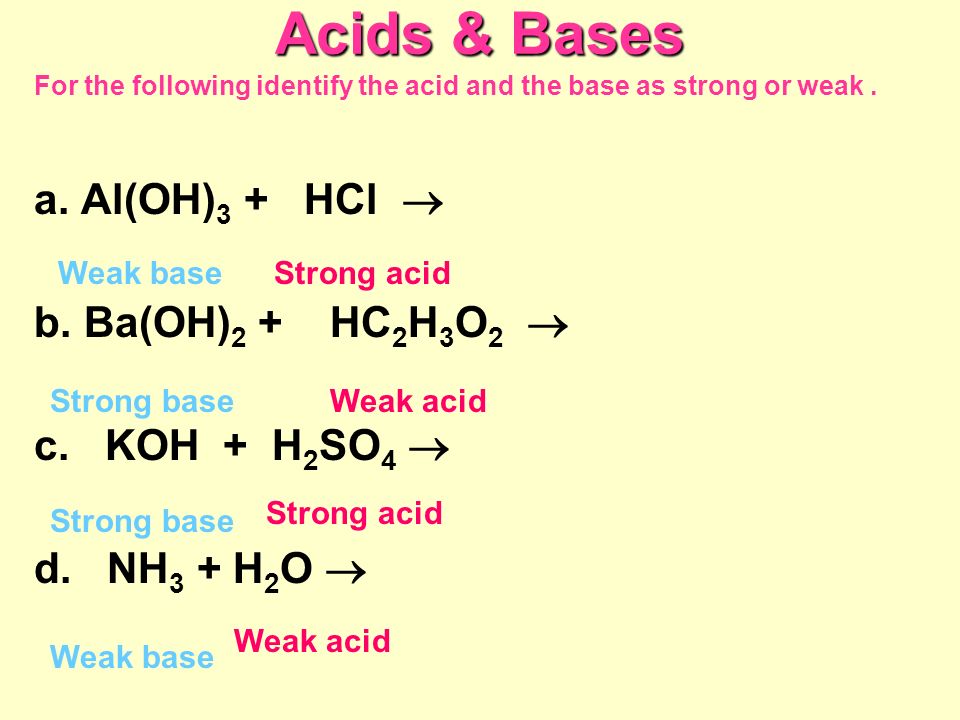
SOLVED: Identify whether each substance is an acid or base. RbOH Choose. HCIO4 Choose . Ba(OH)2 Choose. HNO2 Choose . KOH Choose .

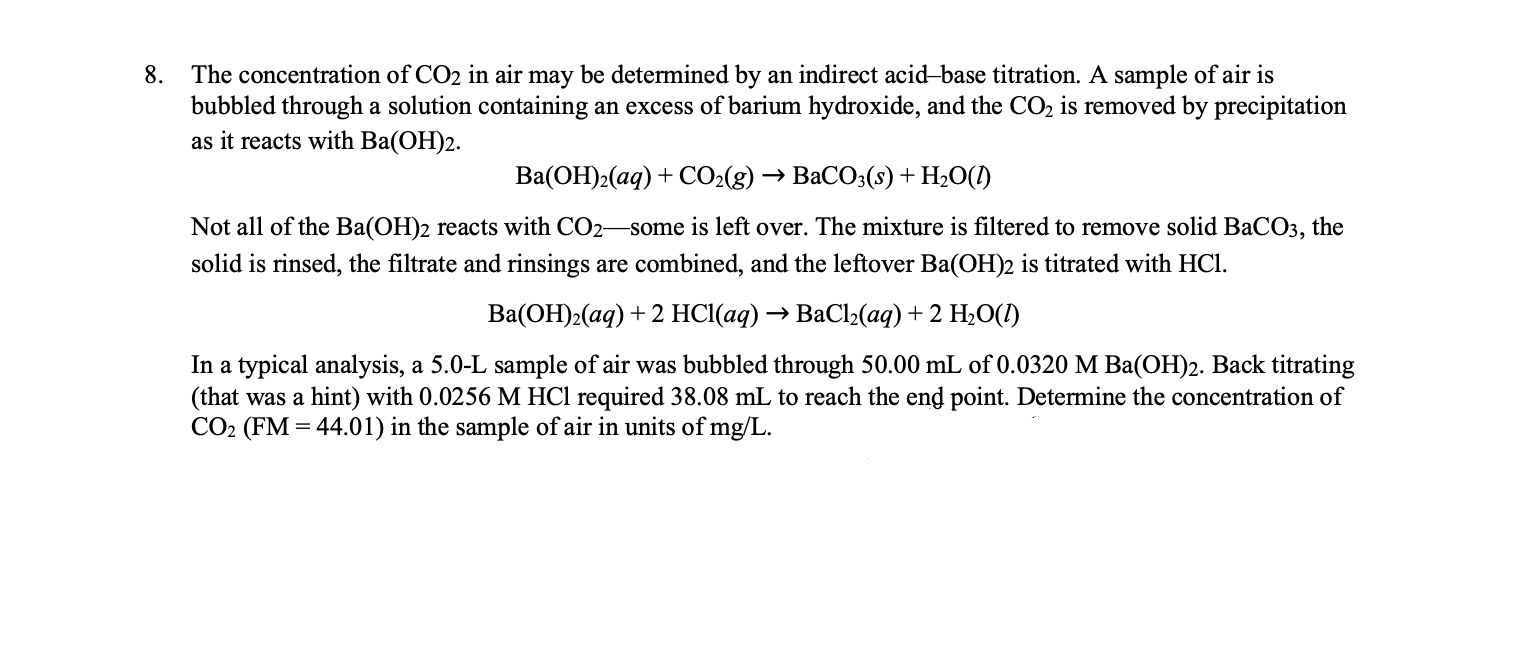
SOLVED: A solution of Ba(OH)2 was standardized against 0.1215 g of benzoic acid with grade of primary standard, C6H5COOH (122.12 g / mol). The end point was observed after adding 43.25 mL

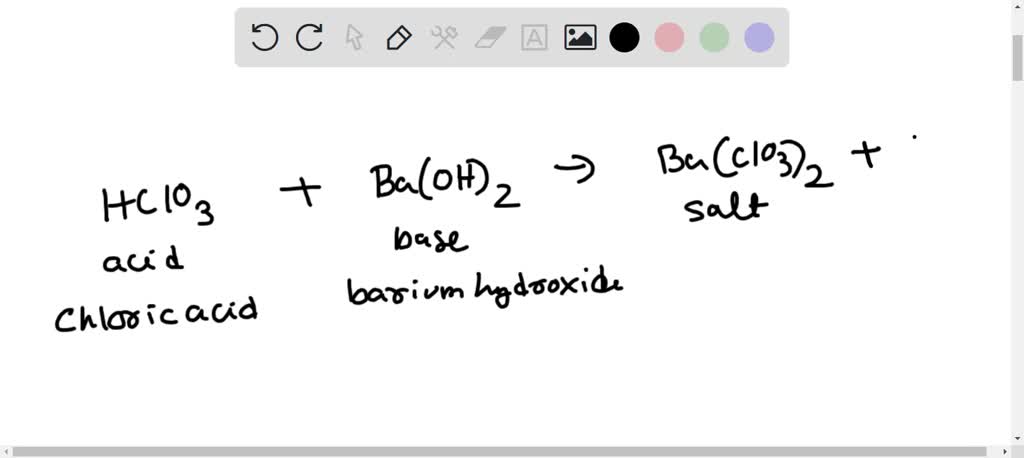
E790: Acid/Base – Conductimetric Titration – Ba(OH)2 + H2SO4 | Lecture Demonstration Manual General Chemistry | University of Colorado Boulder

Is Ba(OH)2 an Acid, Base, or Neutral? (Barium Hydroxide) | Is Ba(OH)2 an Acid, Base, or Neutral? (Barium Hydroxide) Do you want to find out if Barium Hydroxide is an acid, base,

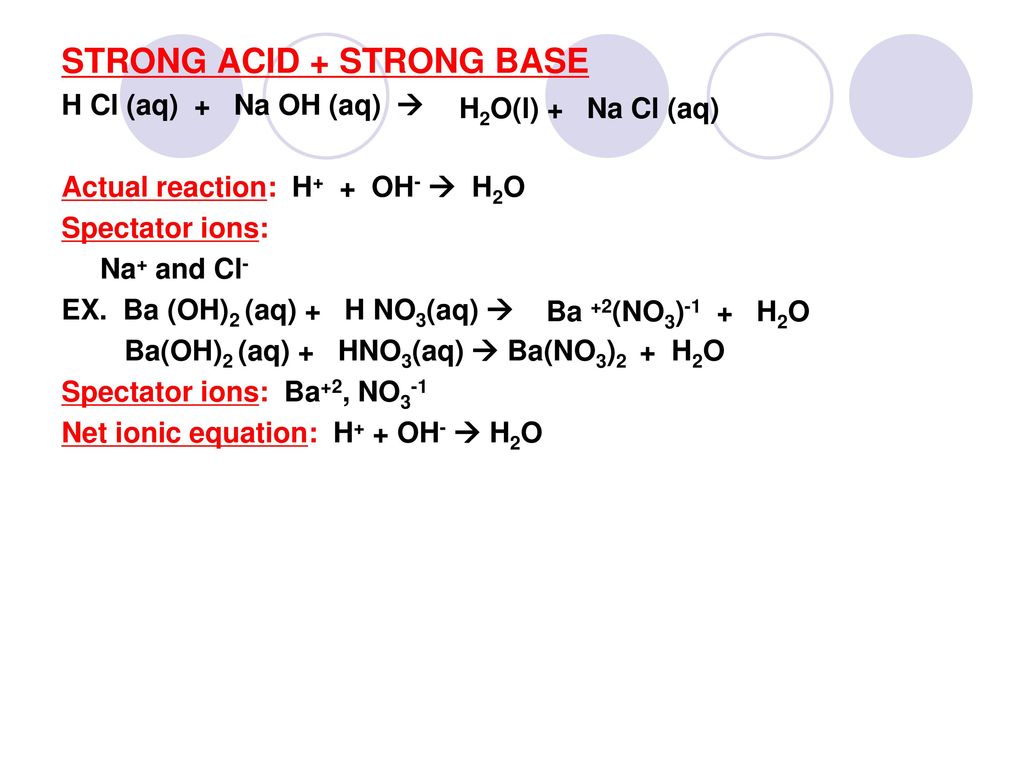
Acids & Bases Acids: acids are sour tasting Arrhenius acid Arrhenius acid: Any substance that, when dissolved in water, increases the concentration. - ppt download